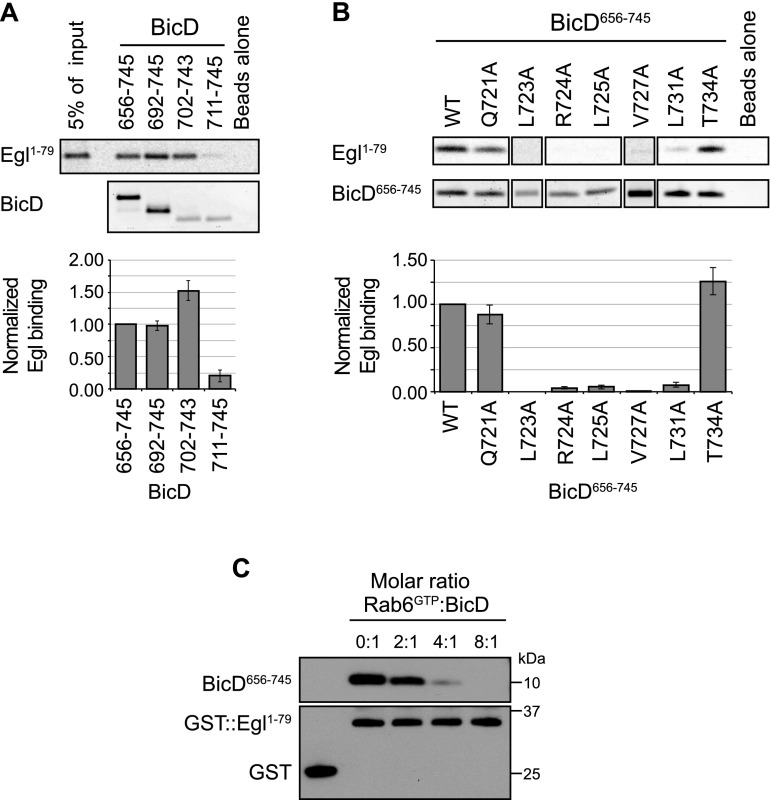
Figure 4.
The Egl-binding site on BicD is highly similar to, or the same as, the binding site for Rab6GTP. (A,B) Representative data (top) and quantification (bottom) of truncation (A) and mutational (B) analysis of the features of BicD important for binding Egl. Egl1–79 was translated in vitro in the presence of 35S-methionine and incubated with His-tagged BicD proteins immobilized on a nickel affinity matrix. Mean values for Egl1–79 binding (generated following correction for variation in BicD signal in each sample and normalized to values for Egl1–79 binding to wild-type [WT] His∷BicD656–745) derive from three experiments for each condition, except for wild type and L731A (six each). Error bars are SEM. (C) Immunoblots from a representative pull-down assay showing that the binding of His∷BicD656–745 to recombinant GST∷Egl1–79 immobilized on a glutathione sepharose matrix is reduced by increasing concentrations of Rab6GTP. BicD656–745 does not bind to GST alone. Rab6GDP does not compete for Egl:BicD binding even at an eightfold molar excess to BicD (Supplemental Fig. S7C). Proteins were visualized with antibodies against His or GST.