Figure 3. Combination of EF24 with SB203580 synergistically induces apoptosis of lung cancer cells.
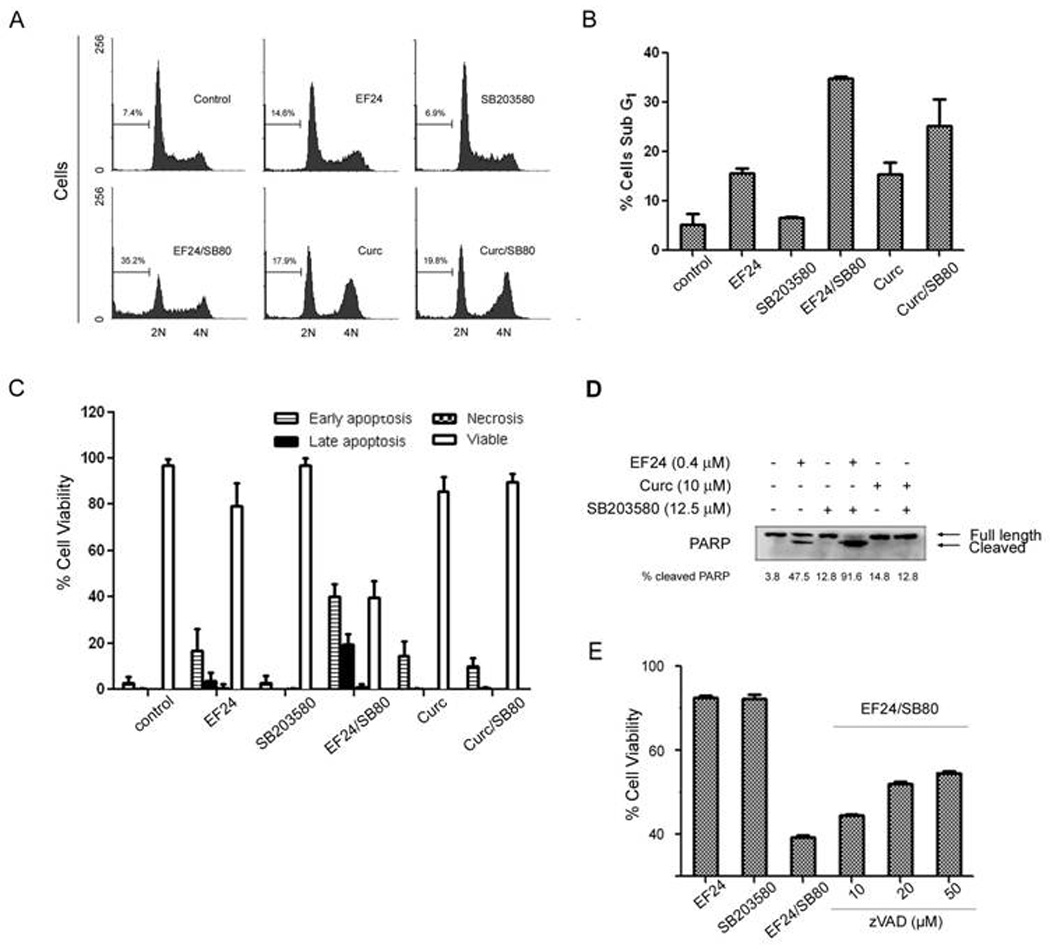
(A) Cell cycle analysis of combined treatment of EF24 (0.4 µM) and SB203580 (12.5 µM). A549 cells were exposed to the indicated compound for 48 hr. Flow cytometry was performed to define the cell cycle distribution based on nuclear content of cells as described in Materials and Methods. The data represent the cell cycle distribution for a representative experiment. (B) Summary of results with sub-G1 cells from three independent experiments. (C) Results of a cell health assay. A549 cells were plated in a 96 well plate at a density of 5000 cells/well. The following day, the cells were treated with the indicated concentrations of EF24, SB203580, Curcumin, or the combinations of EF24 or curcumin with SB203580 for 48 hr. The cellular stains DAPI, Yo-Pro-1, and PI were added to each well. Yo-Pro-1 positive cells are deemed early apoptotic, PI-positive cells are necrotic, and Yo-Pro-1 and PI-positive cells are late apoptotic [29]. Cells that excluded both stains but had intact nuclear staining were deemed viable. A second concentration of EF24 (0.6 µM) served as a control to ensure the assay could identify an increase in apoptotic cells. (D) Western blot analysis of A549 lysates treated with EF24 (0.4 µM), SB203580 (12.5µM), curcumin (10 µM) or the combination of two compounds after 48 hr. The amount of full length and cleaved PARP was revealed by Western blotting using an anti-PARP antibody. (E) Caspase inhibitor effect. A549 cells were pretreated with the caspase inhibitor zVAD-fmk at indicated concentrations for 1 hr before treatment with the combination of EF24 (0.4 µM) and SB203580 (12.5 µM).
