Figure 4.
The Second SH3 Domain of Nck Is Essential for Actin Tail Formation
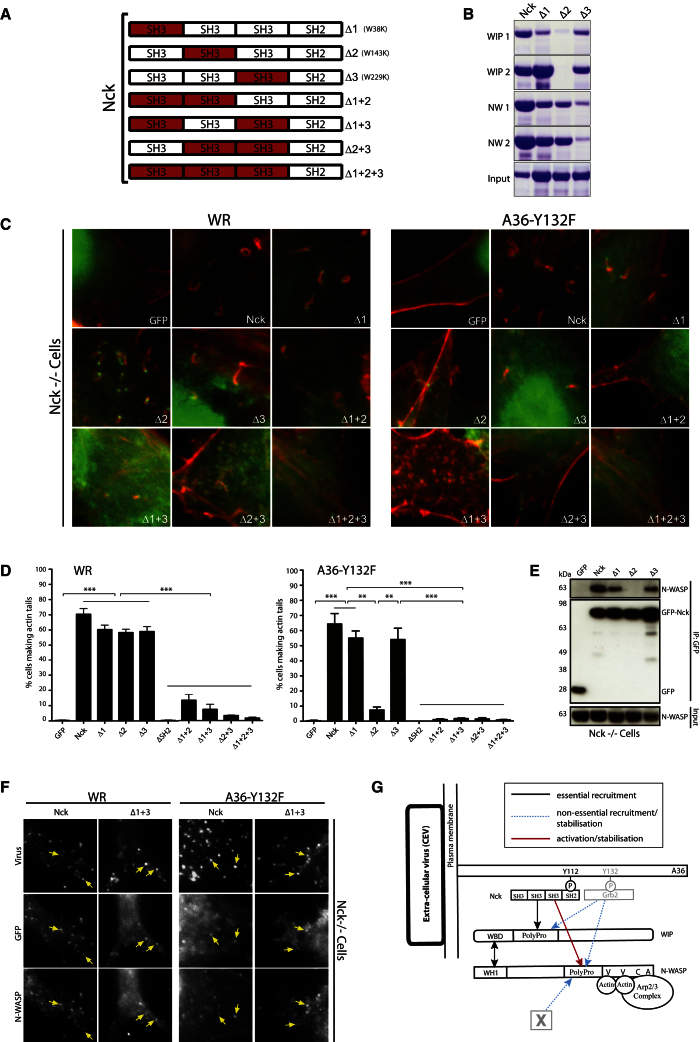
(A) Schematic representation of the SH3-disrupting point mutations (highlighted in red) introduced into Nck.
(B) Pull-down of recombinant His-tagged wild-type or mutant Nck with the indicated peptides (left) identified in the WIP and N-WASP peptide arrays.
(C) Immunofluorescence images of actin tails induced by WR or A36-Y132F in Nck−/− cells expressing the indicated GFP-tagged Nck mutant.
(D) Quantification of the percentage of WR or A36-Y132F-infected Nck−/− cells expressing the indicated GFP-Nck mutant inducing actin tails. Error bars represent the SEM from three independent experiments. **p < 0.01; ***p < 0.001.
(E) Immunoblot analysis demonstrates that endogenous N-WASP does not coimmunoprecipitate with GFP-NckΔ2. The N-WASP input and the immunoprecipitated GFP-tagged proteins are indicated.
(F) Immunofluorescence images demonstrating that endogenous N-WASP is recruited to WR or A36-Y132F viruses in Nck−/− cells expressing the indicated GFP-tagged Nck or NckΔ1+3.
(G) Schematic representation of the interactions and their importance in the Nck:WIP:N-WASP-signaling network. Light gray (Grb2 and X) help but are not essential for Nck:WIP:N-WASP-dependent actin tail formation. Scale bars represent 2 μm. See also Figure S4.