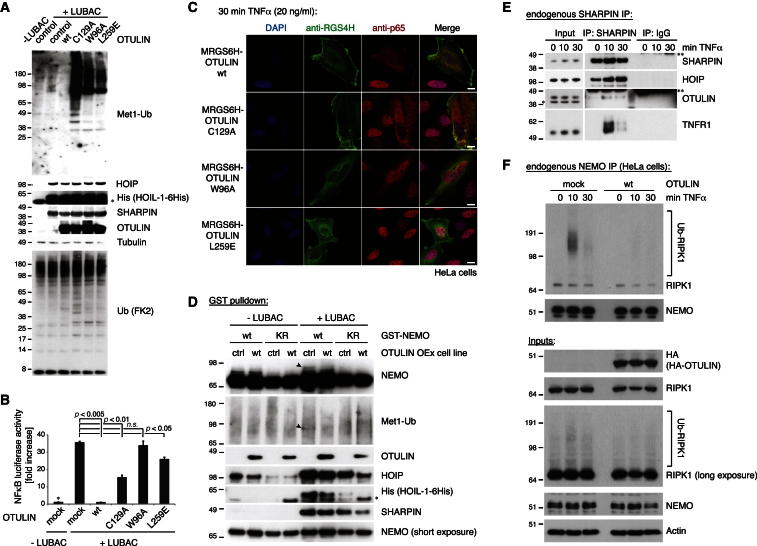
Figure 4.
Cellular Functions of OTULIN
(A) HEK 293ET cells were transfected with plasmids for LUBAC and indicated OTULIN variants (see the Extended Experimental Procedures), and lysates were analyzed by western blotting with the indicated antibodies, including the Met1-linkage-specific antibody (Matsumoto et al., 2012).
(B) NF-κB luciferase assays in HEK 293ET cells for the experiment shown in (A). Error bars represent SD from the mean of experiments performed in triplicate. p values are given to indicate significance. *, mean value set to 1; n.s., nonsignificant.
(C) HeLa cells were transiently transfected with indicated plasmids, treated with TNFα (20 ng/ml) for 30 min, and analyzed by immunofluorescence with indicated antibodies (see Figure S5 for controls and quantification). The scale bar represents 10 μm.
(D) Pulldown of GST-tagged NEMO wild-type (WT) or K285/309R (KR), with or without LUBAC, in control or OTULIN-overexpressing T-REx 293 cell lines. Western blotting with indicated antibodies reveals polyUb on NEMO (arrowhead), which is lost when OTULIN is coexpressed.
See also Figure S5.
(E) Immunoprecipitation (IP) of endogenous SHARPIN coprecipitates HOIP and OTULIN and after TNFα stimulation (100 ng/ml), also TNFR1 is shown as revealed by western blotting with the indicated antibodies. *, nonspecific band; **, heavy chain.
See also Figure S5.
(F) IP of endogenous NEMO coprecipitates polyubiquitinated RIPK1 (Ub-RIPK1) after TNFα stimulation (10 ng/ml) of HeLa cells. Western blotting with the indicated antibodies reveals that transient OTULIN overexpression prevents this complex formation.