Abstract
Class B G-protein-coupled receptors (GPCRs) are receptors for peptide hormones that include glucagon, parathyroid hormone, and calcitonin. These receptors are involved in a wide spectrum of physiological activities, from metabolic regulation and stress control to development and maintenance of the skeletal system. As such, they are important drug targets for the treatment of diabetes, osteoporosis, and stress related disorders. Class B GPCRs are organized into two modular domains: an extracellular domain (ECD) and a helical bundle that contains seven transmembrane helices (TM domain). The ECD is responsible for the high affinity and specificity of hormone binding, and the TM domain is required for receptor activation and signal coupling to downstream G-proteins. Although the structure of the full-length receptor remains unknown, the ECD structures have been well characterized for a number of Class B GPCRs, revealing a common fold for ligand recognition. This review summarizes the general structural principles that guide hormone binding by Class B ECDs and their implications in the design of peptide hormone analogs for therapeutic purposes.
Keywords: G-protein-coupled receptor (GPCR), parathyroid hormone, glucagon, calcitonin, crystal structure
Introduction
GPCRs are cell-surface receptors that share a common molecular architecture consisting of a seven transmembrane (7TM)/ heptahelical domain (HD) with an extracellular N-terminus and an intracellular C-terminus. The seven transmembrane helices are interconnected by three extracellular and three intracellular loops (Figure 1A). All GPCRs share a common signaling mechanism mediated by heterotrimeric G proteins that stimulate the synthesis of intracellular second messengers, including cyclic AMP, inositol phosphate, and Ca2+ ions. GPCRs constitute a large family whose members are involved in numerous physiological functions and represent more than 30% of all pharmaceutical drug targets. Based on sequence homology of their transmembrane domains, G-protein coupled receptors are further classified into five subfamilies1. The Class A or rhodopsin family constitutes the largest group with more than 700 receptors and is characterized by high sequence identity. The Class B or secretin receptor family is a small subgroup with only 15 peptide-binding receptors in humans (Table 1) that are the focus of this review. The other classes are the glutamate (Class C), adhesion (Class D), and frizzled/smoothened (Class E) receptor families2, 3. While all GPCRS share the same core structure to transduce exogenous signals across the membrane, they differ largely in their ligand recognition mechanism due to structural differences in their extracellular domains. Class B GPCRs are characterized by the presence of large extracellular domains of 100 to 160 residues that are the main determinants for ligand binding specificity and play crucial roles in receptor activation4.
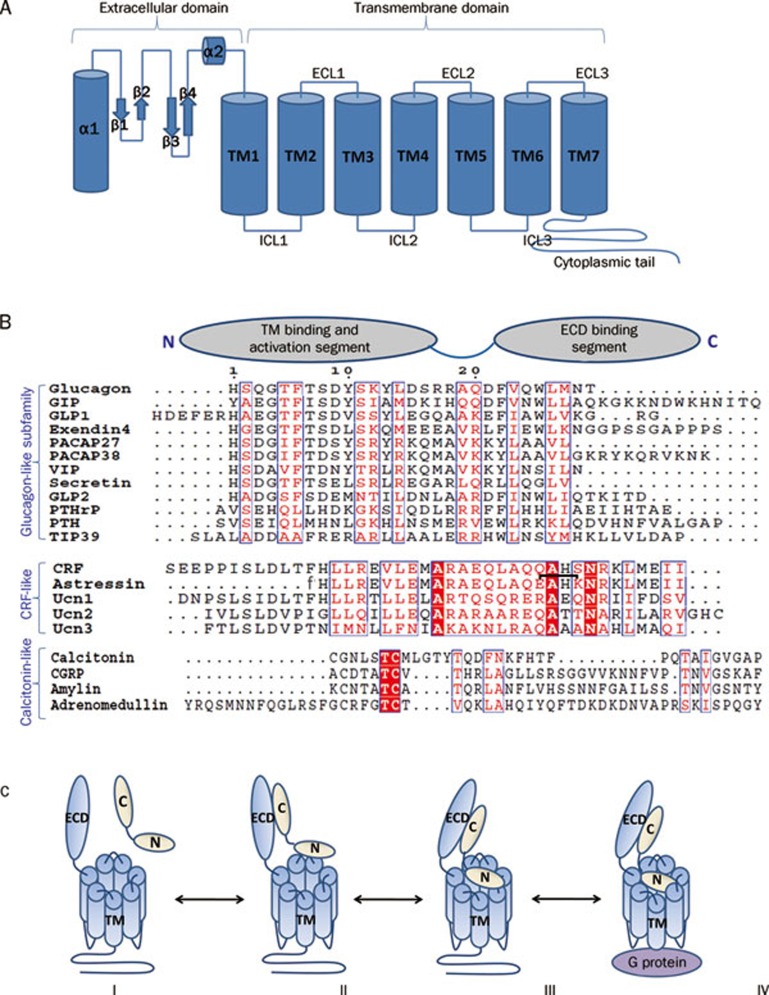
Figure 1.
(A) Cartoon presentation of the general architecture of Class B GPCRs consisting of a N-terminal extracellular domain (ECD) and a C-terminal transmembrane domain (7TM). The ECD forms a three layer α-β-β/α fold and the 7TM domain seven membrane-spanning helices connected by three extracellular loops (ECLs) and three intracellular loops (ICLs). (B) Sequence alignment of Class B GPCR ligands with cartoon presentation of their N- and C-terminal domains on top. Based on sequence similarity, ligands can be grouped into glucagon-like, CRF-like, and calcitonin-like subfamilies. Identical residues are shown as white letters on red background. Partially conserved residues are shown as red letters. The residue numbering on top corresponds to that of glucagon. The lactam bridge in astressin is indicated by a black bracket, “f” in the astressin sequence indicates D-phenylalanine. (C) Two domain binding model for class B GPCRs. (I) Peptide hormone and receptor are orientated for initial receptor ligand binding. (II) The initial complex forms between the C-terminus of the peptide and the ECD of the receptor. (III) This interaction facilitates the binding of the free N-terminus of the peptide to the juxtamembrane region of the 7TM domain of the receptor. (IV) This binding induces a conformational change in the 7TM and cytoplasmic domain of the receptor, which mediates its interaction with a heterotrimeric G protein.
Table 1. An overview of secretin receptor family members and their ligands, with structural details and therapeutic applications.
| Secretin family receptor | Cognate ligands | ECD-PDB | Physiological relevance | Disorders | Therapeutic drugs |
|---|---|---|---|---|---|
| CRFR1 | CRF, Urocortin1 | 3EHS, 3EHU11, 2L2713, | Stress related pathway | Depression/Anxiety | Corticorelin/ Acthrel (approved) |
| CRFR2α | CRF, Urocortin1, Urocortin2, Urocortin3 | 1U345, 2JND6, 3N96, 3N95, 3N9314 | Stress related pathway, Cardiac contractility, Angiogenesis | Depression/Anxiety, Heart failure, Cancer | |
| PTH1R | PTH, PTHrP | 3L2J 39, 3C4M10, 3H3G12 | Ca2+ homeostasis Hyperparathyroidism | Osteoporosis | Forteo (approved) Preotact (approved in Europe) |
| PTH2R | PTH, Tip39 | – | Hypothalamic secretion, Nociception | ||
| GHRH receptor | GHRH | – | Growth hormone secretion | Dwarfism | Tesamorelin (approved) |
| Glucagon receptor | Glucagon | – | Glucose homeostasis | Type 2 diabetes | |
| GLP1 receptor | GLP-1, Exendin4 | 3IOL76, 3C599 | Insulin secretion | Type 2 diabetes | Byetta/Exenatide, Liraglutide (both approved) |
| GLP-2 receptor | GLP-2 | Glucagon secretion, Gut mucosal growth | Short bowel syndrome | Teduglutide (Phase III) | |
| GIP receptor | GIP | 2QKH7 | Insulin secretion Lipid metabolism | Type 2 diabetes | – |
| PAC1R | PACAP, VIP | 3N9416, 2JOD8 | Neurotransmitter, Neuromodulator, Neuroprotection | Schizophrenia, Medulloblastoma | – |
| VPAC1R | VIP, PACAP | – | Vasodilation, Digestion, Neuroprotection | Crohn's disease, rheumatoid arthritis | – |
| VPAC2R | VIP, PACAP | 2X57 | Vasodilation, Digestion, Neuroprotection | Schizophrenia | – |
| Secretin receptor | Secretin | – | Pancreatic secretin H2O homeostasis | – | |
| Calcitonin receptor | Calcitonin | - | Ca2+ homeostasis | Osteoporosis | Miacalcin, Cibacalcin (approved) |
| AMY receptor (CTR /RAMP1,2,3) | Amylin | Glucose homeostasis | Diabetes | Pramlintide/Smylin (approved) | |
| CGRP receptor (CLR/RAMP1) | CGRP | 3N7S15 | Vasodilation | Migraine | Telcagepant (Phase III fail) |
| AM1 receptor (CLR/RAMP2) | Andromedulin | 2XVT (RAMP2) | Circulatory system, Vasodilation | Cardiovascular disease | |
| AM2 receptor (CLR/RAMP3) | CGRP, Andromedulin | Vasodilation, Cellular tolerance for oxidative stress | Cardiovascular diseases | – |
Although full length GPCR structures have been solved only for Class A receptors, the structures of several Class B extracellular domains (ECDs), both in apo and hormone-bound form, have been determined by X-ray crystallography and NMR5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16. These structures have provided substantial information about the conformation of Class B ECDs and the structural mechanisms of ligand binding and selectivity. Table 1 provides a list of the currently solved 16 ECD structures that collectively cover eight of the fifteen receptors.
Class B GPCR ligands are related peptide hormones
In contrast to the wide variety of Class A GPCR ligands, all Class B ligands are peptide hormones that share significant degrees of homology with each other (Figure 1B). All of them have great potential as therapeutic targets for neuronal and endocrinal disorders.
Secretin is the sole ligand of the secretin receptor. It stimulates secretion of acid-neutralizing fluids in pancreas and duodenum. The expression of the secretin receptor in different parts of the CNS imply that secretin also plays important roles in the brain17.
Growth hormone releasing hormone (GHRH) is the sole hormone of the GHRH receptor and stimulates growth hormone secretion.
Corticotrophin release factor (CRF) and urocortins are ligands for CRF receptors 1 and 2 (CRFRs) and function predominantly as mediators of stress responses18. Urocortin 2 also has antiangiogenic activity important for tumor suppression function19.
Parathyroid hormone (PTH), parathyroid hormone related peptides (PTHrPs), and tuberoinfundibular peptide Tip39 are ligands for PTH receptors (PTH1R and PTH2R) that control calcium and phosphate homeostasis20 and can function as neuromodulators21.
Glucagon, glucagon like peptides (GLPs), and glucose dependent insulinotropic polypeptide (GIP) are ligands for glucagon receptor (GLPRs and GIPR), respectively, and are important regulators of glucose homeostasis22, 23.
Pituitary adenylate cyclase activating polypeptide (PACAP)and vasoactive intestinal peptide (VIP) are shared ligands of three receptors, PAC1R, VPAC1R, and VPAC2R. PAC1R is preferentially activated by PACAP, a neuroprotective modulator and stimulator of nerve cell regeneration, while VPACR is efficiently activated by both the vasodilation-stimulating VIP and PACAP. VIP also performs neuroprotective function with VPAC2R. Both hormones also have neurotransmitter function and affect secretion or production of other hormones24.
Calcitonin (CT), calcitonin-gene-related peptide (CGRP), amylin (AMY), and andromedullin (AM) form a separate subclass of Class B hormones with roles in Ca2+- and glucose-homeostasis as well as vasodilation. Their receptors, calcitonin receptor (CTR) and calcitonin-like receptor (CLR), associate with three members of receptor activity-modifying proteins (RAMP1 to RAMP3) that modulate their hormone selectivity25.
In addition, two non-human peptide ligands, Exendin-4 and Astressin, have pharmacological roles in treating type-2 diabetes and stress related disorders. Exendin-4 is derived from the saliva of Gila monster. As an analog of GLP-1, it activates GLP1R and stimulates glucose-dependent insulin secretion26. Astressin is a synthetically designed high affinity antagonist for CRFR, in which D-Phe replaces L-Phe at the 12th position of CRF (12–41). In addition, lactam cyclization between astressin Glu30 and Lys33 stabilizes helix formation and strongly increases the affinity of the peptide.
Crystal structures of Class B GPCR ligands7, 27 revealed single continuous amphipathic α-helices, while NMR solution structures28, 29 indicated that the free peptide hormones are disordered or only partially α-helical, but adopt amphipathic α-helices upon receptor binding. Binding studies employing truncated and chimeric peptide ligands demonstrated separate contributions by the peptide N- and C-termini. N terminal truncations turn the peptide ligands into potent antagonists24, 30, suggesting that the N-termini play critical roles in receptor activation, but are not essential for receptor binding. In contrast, C-terminally deleted peptides are still capable of receptor activation, but bind the receptors with markedly lower affinities31. Finally, peptides consisting of the C-terminus of PTH and the N-terminus of calcitonin were unable to activate either PTHR or CTR, but efficiently activated a chimeric receptor consisting of the N-terminal ECD from PTH1R and the membrane embedded C-terminus from CTR32. Several other similar hybrid experiments with glucagon, GLP1R, calcitonin, VIP, and PACAP confirmed the distinct roles of the N- and C-termini of the peptide hormones in receptor interaction and activation30, 31, 33, 34, 35. These data provided the basis of the “two domain model”, which proposes that Class B hormone C-termini form initial complexes with their receptor ECDs, which in turn allows their N-termini to interact with the 7TM domains to activate the receptors (Figure 1C). This model was further supported for CRF by NMR chemical shift perturbation data in combination with the charge distribution in CRF ECD and its antagonist astressin5. Finally, the first high resolution structure of the complex between a Class B GPCR ECD and its ligand, the crystal structure of the GIPR ECD–GIP (1–42) complex, directly illustrated that the C-terminus of the ligand formed the main ECD interaction while the N-terminus of the peptide remained free7.
The peptide hormone N- and C-termini expressed as separate peptides are biologically inactive, implying that their linkage is required for hormone activity36. The residues that connect the termini appear to function as α-helical linkers, whose length and orientation, but not sequence, are required for full receptor activation36.
The extracellular domains of Class B GPCRs share a common fold
While the 7TM domains of Class B GPCRs are highly homologous, their ECDs share exceptionally low levels of sequence identity (Figure 2B). Therefore, structural studies of ECDs are crucial to understand ligand specificity and selectivity. Table 1 provides an overview of the 15 human Class B receptors and their ECD structures and functions.
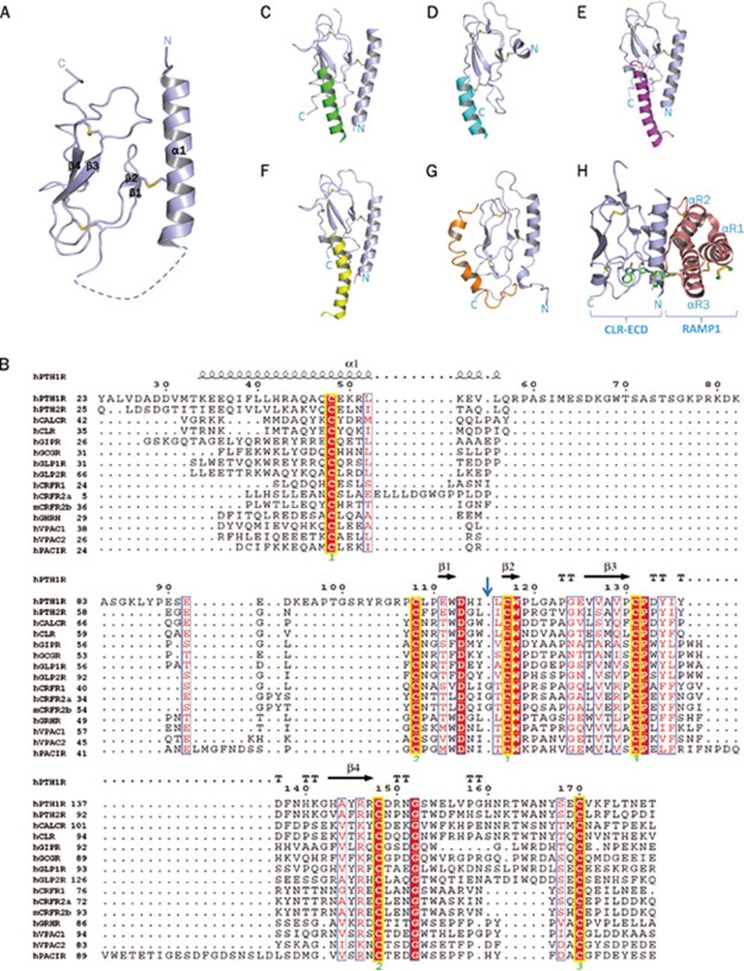
Figure 2.
(A) A ribbon diagram of the basic architecture of the “secretin family recognition fold” of the extracellular domain of class B GPCRs. The structure is mainly divided into three layers consisting of an N terminal α helix and two pairs of antiparallel β sheets. The conserved disulfide bonds connecting the three layers are depicted as sticks. (B) Sequence alignment of the extracellular domains of human Class B GPCRs with secondary structure elements for PTH1R indicated on top (PDB: 3C4M). Invariant and conserved residues are highlighted. The glycine residues specific for the CRFR subfamily are marked by a blue arrow. Invariant cysteine residues are indicated by a yellow box. Identical residues are shown as white letters on red background. Partially conserved residues are shown as red letters on white background. The residue numbering on top corresponds to that of hPTH1R. Cysteine pairs forming disulfide bonds are indicated by yellow outlines and by green numbers at the bottom. TT=tight turns. (C) Structure of the hPTH1R-PTH ECD complex with the ECD shown in light blue and PTH in green. (D) Structure of the hCRFR1-CRF complex with the ECD shown in light blue and CRF in cyan. (E) Structure of the hGIPR-GIP complex with the ECD in light blue and GIP in magenta. (F) Structure of the hGLP1R-Glp1 complex with the ECD in light blue and Glp1 in yellow. (G) Structure of the PAC1R-PACAP complex with the ECD in light blue and PACAP in orange. (H) Structure of the CLR-Telcagepant (a small molecule drug for the treatment of migraine) with the CLR-ECD in light blue and RAMP1 in salmon.
The basic fold of the extracellular domain is a three-layer α-β-β/α structure
The first structures of the extracellular domain of a Class B GPCR were the NMR structures of the ECD of murine CRFR2β in apo form5 and as complex with the synthetic antagonist astressin6. These structures revealed the core region of the ECD, which is comprised of two pairs of antiparallel β-sheets interconnected by hairpin loops. This fold is stabilized by three interlayer disulfide bonds and by hydrophobic interactions and resembles the short consensus repeat fold of complement control protein37. However, the N-terminus of the ECD was not resolved in these structures. The ligand-bound ECD crystal structures of hGIPR–GIP (1–42) and hPTH1R–PTH (15–34) (Figure 2E & 2C) demonstrated that the N-termini of their ECDs form long single α-helices that are connected by a disulfide bond with the first β strand and whose residues contribute to the ECD-ligand binding pocket7, 10. Overall, the ECDs share a three-layer α-β-β/α architecture, in which the N-terminal α-helix forms the first outer layer, the β1-β2 sheet and adjacent loops the middle layer, and the β3-β4 and the C-terminus, which for some ECDs includes a short (one-to-two turns) α-helix, the second outer layer (Figure 2A and 2C–2H). This organization, together with the principal hormone recognition mechanism, has also been found in the hGIPR–GIP, hPTH1R–PTH, hGLP1R–GLP1, and hCRFR1–CRF (Figure 2C–2G). This conserved α-β-β/α fold has also been named 'secretin family recognition fold' that serves as the consensus mechanism of Class B GPCR ligand binding16.
The sequence homology of the ECDs is very low and limited to the six disulfide-forming cysteines and only about a dozen other conserved residues (Figure 2B). Four of the residues are identical in all receptor ECDs and have been shown to play important roles in tertiary structure stabilization (D113, W118, P132, and W154 with respect to PTH1R in the alignment shown Figure 2B)10. The first disulfide bond links the N terminal helix to the middle layer β-sheet, the second one links the middle layer to the outer β-sheet, and the third one links the middle layer to the C-terminus of the ECD. While the position of the disulfide bonds and secondary structure elements is highly conserved, the loops connecting the structure motifs vary considerably and therefore likely provide the basis for ligand binding specificities.
Glucagon-like and CRF-like hormones adopt different positions in their ligand-binding pockets
The overall binding pattern of Class B GPCR peptide ligands to their cognate receptors shows a high level of similarity. In all complex crystal structures, the peptide binds in amphipathic α-helical conformation to the same face of the ECD. With the exception of the PAC1R-PACAP8 NMR structure, whose accuracy remains in doubt16, 38, the C-terminus of the peptide forms hydrophobic and hydrogen bond interactions with the ligand binding pocket of the ECD while the N-terminus of the peptide remains free and shows a high level of flexibility. The glucagon-like and the CRF-like subfamilies differ from each other both by their amino acid sequence signature (Figure 1B) and by their relative position in the ligand binding pocket. As illustrated in Figure 3A, the position of the CRF C-terminal helix is translationally shifted by 5–8 Å relative to the location of PTH, GIP, and GLP. These differences can be explained by two features. First, the CRFR ECDs are characterized by one invariant glycine (Gly52 for CRFR1; blue arrow in Figure 2B) in the loop connecting β1 and β2 that is missing in peptide hormones of the glucagon-like subfamily and that causes a further extension of the loop. Second, the CRFR N-terminal helices of the ECD are much shorter than those of PTHR, GIPR, and GLPR (Figures 2D–2F) and that can therefore not mediate ligand interactions11. It should be noted, that differences in ligand position could also be induced by experimental approaches.
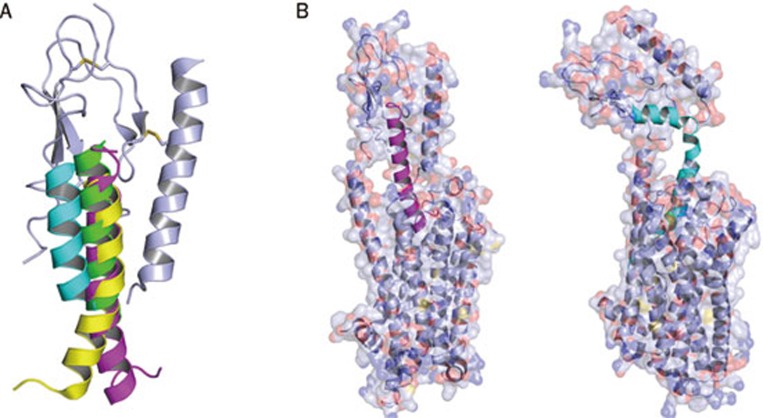
Figure 3.
(A) Structural alignment of ECD-bound Class B GPCR ligands. The ligands form helical conformations with their C termini interacting with the ECD. The N termini remain free and show a high level of flexibility. The ligands shown are PTH(15–34) in green, GIP(1–42) in magenta, GLP1(7–37) in yellow, and CRF(22–41) in cyan. (B) Models displaying possible hormone positions in the context of full length receptors. Models of ECD-bound GIP(1–42) (magenta) [PDB: 2QKH] and a modified CRF (cyan) [PDB: 2L27] were superpositioned on a model of the transmembrane domain of turkey β1-adrenergic receptor [PDB: 2Y03]. The ECD was adjusted manually with distance constraints using COOT. The different binding positions between CRF-like and glucagon-like subfamily peptides suggest that the ECDs may also adopt two different conformations in the context of full length receptors. Note that these models just illustrate relative dimensions of receptors and ligands as well as predictions of ligand binding sites. Only structures of the complexes between full length receptors and their ligands can provide accurate position and conformation of receptor-bound ligands.
Based upon the ligand-bound ECD structures, we illustrate possible models for the binding of these two hormone subclasses to a full length receptor (Figure 3B) to provide the relative dimensions of ligands and receptors and to illustrate the burial of the ligands. In the GIP model, the N-terminus of the ligand forms a straight helical extension of the C-terminus to fit into the pocket formed between the 7TM helices. The CRF model accounts for the L-shaped conformation with a bend after the 23rd residue seen in the recent NMR structure of hCRFR1-CRF (PDB: 2L27)13. Thus, ligands may adopt different positions in spite of a common structural interaction mechanism. The exact nature of complete ligand binding can only be determined by structural analyses of full length receptors.
Ligand-binding induces conformational changes in some ECDs
Most ECD structures have only been obtained in complex with ligand, indicating that ligand binding stabilizes these proteins and therefore favors crystallization of ECD-ligand complexes14. Hormone-induced conformational changes in the ECD have been clearly shown for CRFR111, where superposition of apo (3EHS) and CRF-bound structures (3EHU) revealed a major rearrangement of the ECD secondary structure. Ligand binding shifted the loop connecting the β3 and β4 strands by approximately 5–7 Å towards the peptide hormone. Phe72 in that loop shifted in the ligand bound structure by 7.2 Å and its side chain rotated towards the C terminus of the peptide to allow formation of a hydrophobic interaction. These changes illustrate the dynamic plasticity of the CRFR1 ECD. It will be interesting to see, if similar conformational rearrangements also occur in other ECDs of this class.
In contrast to CRFR1, no major conformational change was detectable between the apo and PTH-bound structure of PTH1R, a representative of the glucagon-like subclass of hormones39. Instead, the long second (C terminal) α-helix of the apo PTH1R ECD mimicked the structure of the peptide hormone in the complex structure.
Peptide ligands can modulate the monomer–dimer equilibrium of Class B GPCRs
Receptor oligomerization has been demonstrated for different classes of GPCRs40. In the case of Class C GPCRs, homo- or hetero-dimerization mediates receptor activation41. Dimerization has also been demonstrated for Class A GPCRs42, 43, including the real-time imaging of muscarinic acetylcholine receptor dimerization in live CHO-cells by total internal reflection fluorescence microscopy (TIRFM)44. Based upon several functional studies, Class B GPCRs can dimerize/oligomerize via their heptahelical domains. In particular, the lipid-exposed hydrophobic surface of their TM4 helices appears to mediate receptor homo-dimerization45, 46. The functional significance of Class B GPCR oligomerization is poorly understood. In the case of the secretin receptor, disruption of the interaction between tagged receptors in cells had no effect on ligand binding, but did reduce receptor signaling by an unknown mechanism45. In addition to homo-oligomerization, hetero-oligomer formation has also been observed between VIP receptors VIP1R/VPAC2 and the secretin receptor47 as well as for calcitonin receptor48, CRFR49, and PAC1R50.
The ligand-bound structures of Class B GPCR ECDs mostly presented monomeric conformations. The exception is the PTH1 receptor, whose ECD adopted a dimeric conformation in the absence, but not the presence, of ligand. This result is consistent with bioluminescence resonance energy transfer (BRET) analysis of full length PTH1R, which demonstrated that addition of ligand leads to disruption of receptor dimers39. The dimeric arrangement of protomers in the apo ECD was mediated by the C-terminal α2 helix, which occupied the peptide binding groove of the other monomer. In the absence of ligand, the C-terminal helix thus structurally mimics the ligand which leads to the dimer formation. Dimer formation was validated by BRET experiments with receptors in which the α2-helical region of the ECD was mutated. While these experiments provided a structural basis for ECD-mediated receptor dimerization for PTH1R, this feature may not be shared among other members of secretin family receptors.
Class B peptide ligands adopt α-helical conformations upon receptor binding
In complex with their receptor ECDs, peptide hormones are α- helical in both crystal and solution structures. In most cases, the helices were amphipathic, especially at the C terminus, which is the main determinant for ECD binding. In contrast, the free peptides appear to be unstructured in water and adopt their helical structure upon complex formation. The thermodynamic and spectroscopic analysis of GIP peptide upon binding to GIPR revealed the burial of a solvent accessible region and an increase in α helical structure, which contributes to an increase in receptor affinity and the formation of a tight hormone-receptor complex7. Using NMR techniques, a transition from an unstructured to an α-helical conformation was also observed for the binding of CRF to its ECD in an analysis of the minimum peptide length requirement for ECD binding51. These results agree with a comparative NMR analysis of the conformational changes in PACAP (1–27) upon association with its full length receptor in micelles52.
The helix-capping residues at the N termini of Class B hormone ligands play a crucial role in initiating the transition to an α-helical conformation53. Functional studies using α-aminoisobutyrate analogs of PTH have also shown that the N terminal region forms a helical conformation when complexed with the extracellular loops and TM domains of PTH1R54.
Many ECDs can interact with different ligands
Selectivity within the glucagon-like and CRF-like subfamilies is determined by non-conserved amino acids
Most Class B GPCR can bind to more than one ligand. For example, PAC1R has a very high affinity for PACAP [both PACAP(1–38) and (1–27)], but can also interact with VIP. Early demonstration of glucagon/GLP1 selectivity was achieved by the use of chimeric GLP1 and glucagon receptors30, 31.
The family of CRF/Ucn peptides signals through two different receptors, CRFR1 and CRFR2. Although these two receptors share 68% sequence identity, they differ markedly in ligand selectivity. CRFR1 is selective for CRF and Ucn1 while CRFR2 binds all four ligands CRF, Ucn1, Ucn2, and Ucn3 with affinities that range from high to moderate. Structural and biochemical analysis of the binding of the CRFR ECDs to the C termini of CRF, Ucn1, Ucn2, and Ucn3 identified the selectivity determinants that distinguish between the highly similar peptide hormones. The importance of these critical residues responsible for selectivity was confirmed by swapping experiments. Ucn1 and CRF contain an arginine at position 35 that is missing in Ucn2 and Ucn3 and that may determine selectivity to CRFR1 by binding to a CRFR1-specific negatively charged pocket consisting of Gln103 and Glu10414. Importantly, the affinity and selectivity patterns of the ECDs closely match those of the full length receptors55. Thermodynamic and CD analyses of CRF and urocortins may help to evaluate whether an inherent high helical propensity is responsible for the high affinity binding of Ucn1 (relative to CRF and Ucn2/3) to both CRFRs.
The structural determinants of the selectivity of PTHRs are still unclear. The three ligands PTH, PTHrP, and TIP39 mediate their biological functions through two receptors, PTH1R and PTH2R. PTH can bind both receptors, while PTHrP is selective for PTH1R and TIP 39 is selective for PTH2R56. Radioligand binding studies with wildtype and chimeric PTH2R/PTH1R receptors have pointed to the N terminal six residues of the ligand and to the extracellular loops of the TM domains as important selectivity determinants57. In addition, the presence of Trp2358, a residue that is invariant in both PTH and TIP39, is probably responsible for selective binding of these two ligands to PTH2R.
Importantly, binding of different ligands can induce highly distinct pharmacological receptor responses. This was first shown for CRFR, where different physiological ligands and CRF receptor subtypes can differentially stimulate signaling pathways in human myometrial cells59, effects that could only partially be confirmed in other cell types60. Many GPCRs can signals through β-arrestins in addition to the classical G protein pathways61 and certain PTH analogs have been shown to function as “biased agonists” that preferentially signal either through G proteins or through β-arrestins62, 63.
Radioligand dissociation experiments with full length PTH1R and G proteins have shown that PTH (1-34) has a higher affinity than PTHrP (1–36) for PTH1R in its G protein-uncoupled conformation (R0 state), while both peptides bind PTH1R with equal affinities in its G protein-coupled state (RG). Since the ECD adopts the same conformation when bound to either PTH or PTHrP, it is likely that this selectivity is due to ligand-selective rearrangements in the heptahelical domain of the receptor. Therefore, full length structures of PTH1R with PTH and PTHrP will be required to provide a structural basis for the ligand selectivity of the R0 and RG states of the receptor. Analysis of differential ligand binding to different states of receptor may significantly contribute to the development of specific peptide analogs for therapeutic purposes64 (see below).
Selectivity of the calcitonin subfamily of receptors is modulated by RAMPs
At a different level of selectivity, the calcitonin receptor CTR and the calcitonin-like receptor CLR form heterodimeric complexes with transmembrane protein partners called receptor activity modifying proteins (RAMPs). The complex between CLR and RAMP1 is selectively bound and activated by the calcitonin gene-related peptide (CGRP), while CLR in complex with RAMP2 and RAMP3 form adrenomedullin AM1 and AM2 receptors, respectively. In the absence of RAMPs, CTR is preferentially activated by calcitonin, whereas it functions predominatly through amylin (AMY) in combination with RAMP1, 2, and 365. The functional association of several other Class B members with RAMPs has been reported previously, but the functional role of these complexes remains unknown25.
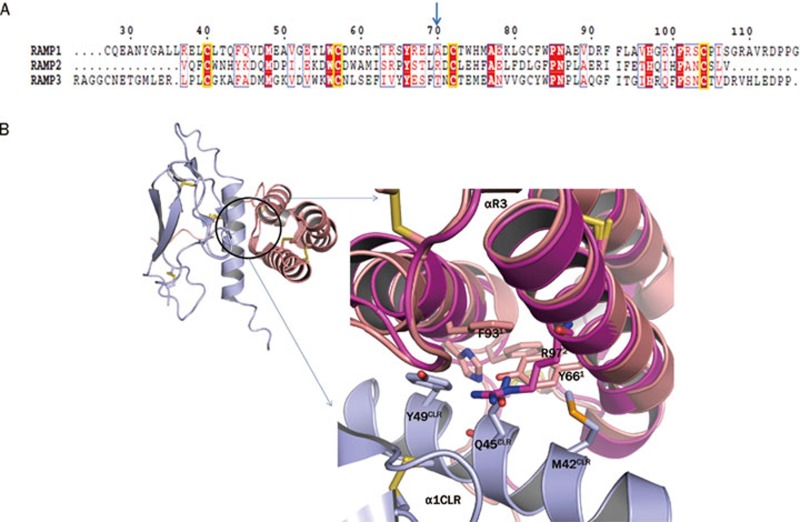
The first crystal structure of RAMP1 (PDB: 2YX8) revealed a triangular arrangement of a three helix bundle that is stabilized by three interconnecting disulfide bonds formed between six invariant cysteine residues (Figure 4A)66. The subsequent crystal structure of RAMP1 in complex with the extracellular domain of CLR (PDB: 3N7P, 3N7R, SN7S) identified both hydrophobic and electrostatic interactions between the R2 and R3 helices of RAMP1 and the N terminal helix of the CLR ECD15. The main RAMP residues that form hydrophobic interactions with CLR are the conserved Tyr66, Phe93, His97 and Phe101 (numbering refers to RAMP1, see alignment in Figure 4A). Based upon the high sequence and structural conservation (root mean square deviation of 1.24 Å) between RAMP1 and RAMP2, similar interactions are predicted for the CLR-RAMP2 complex. However, structural alignment of the CLR-RAMP1 complex (PDB: 3N7S) with apo RAMP2 (PDB: 2XVT) reveals a sterical clash between the side chain of Arg97 in the second helix of RAMP2 (RAMP1 has an alanine at the corresponding position) and the side chain of CLR Gln45 (Figure 4B), suggesting that RAMP2 may structurally rearrange when forming the ternary CLR-RAMP2-AM complex.
Figure 4.
(A) Sequence alignment of RAMP 1, 2, and 3. The important non-conserved residue in the RAMP1-CLR interaction pocket has been highlighted by a blue arrow. Invariant cysteine residues have been shown in yellow box. Identical residues are shown as white letters on red background. Partially conserved residues are shown as red letters. The residue numbering on top corresponds to that of RAMP1. (B) Structural representation of the CGRP receptor with the CLR-ECD in light blue and RAMP1 in salmon. Structural alignment of the binding interface of the RAMP1-CLR complex (salmon-lightblue; PDB: 3N7S) with apo RAMP2 (magenta; PDB: 2XVT). The helices 2 and 3 of RAMP have been marked. The side chains of RAMP2 R97 and CLR Q45CLR in the binding interface sterically clash.
Structure-based chemical modification of peptide ligands is important for therapeutic applications
The potential for therapeutic applications of Class B GPCRs and their peptide binding partners is enormous. However, direct application of these peptides as therapeutic drugs is hampered by their low efficacy due to poor bioavailability and rapid degradation. Therefore, a substantial amount of research is dedicated to the design of stable, chemically modified analogs of these peptides67. Modifications used to increase peptide stability include (i) N-terminal fatty acid acylation (GLP1) or hexonylation (VIP), (ii) generation of chimeric hormones (Glp1/PACAP fusions), (iii) midchain modifications by mercaptopropionic acid derivatization of Cys14 in andromedulin and by replacing L-Phe12 in CRF with D-Phe, as well as (iv) alteration at the C-terminus by PEGylation (GIP)68, 69, 70, 71, 72. The resulting agonists and antagonists have increased metabolic stability, biological activity, and bioavailability. For example, the energy metabolism-regulating hormone Glp1 is highly unstable with a bioavailability of only 1–2 min due to its rapid enzymatic degradation by dipeptidyl peptidase 4. In contrast, the N-acylated Glp1 analog Liraglutide has a half life of 14 h, which makes it suitable for the treatment of type 2 diabetes73. Exenatide, an analog of the naturally occurring Glp1 agonist Exendin-4, in which the second alanine is substituted by serine, improves both stability and activity73. Exenatide increases insulin secretion in response to high blood sugar levels and suppresses the pancreatic release of glucagon. Similarly, a tetra-substituted analog of GHRH (1–29) circumvents proteolytic cleavage by associating with serum albumin74. Introduction of two proline residues at the very N-terminus of PTH (1–34) generated an analog that is resistant against degradation by dipeptidase and that is currently in use for the treatment of osteoporosis75.
Conclusions
The structures of the ECDs of Class B GPCRs in apo and hormone-bound forms have identified the main determinants of receptor-peptide specificity and have established the unique fold of the extracellular receptor domains and the conserved conformation of their bound peptide ligands. The structural data are consistent with various previous in vivo functional studies. Therefore, in the absence of full length receptor structures, ECD-hormone complexes provide the best model for rational drug design and further studies. There remains an ongoing need for structural interrogations of ligand-receptor specificities and selectivities for the design of more precise, specific, and stable peptides as therapeutic drugs to treat the many diseases impacted by Class B hormones.
Crystallization of full length Class B GPCRs may provide an even more formidable challenge than crystallization of Class A GPCRs due to the presence of the Class B-specific long flexible N terminal extracellular-domains. The main bottleneck for crystallization of GPCRs has been their conformational flexibility. This bottleneck could be overcome for several Class A GPCRs by protein engineering approaches, including the introduction of stability-enhancing mutations, replacement of flexible surface loops with stable proteins like T4 lysozyme, and stabilization by complex formation with nanobodies. In addition, improvements in the lipidic cubic phase method of membrane protein crystallization and in data collection using micro beam technology have further stretched the boundaries of membrane protein crystallization. Given these technical advances, we look optimistically into the future of Class B GPCR crystallography.
Acknowledgments
This work was supported in part by the Jay and Betty Van Andel Foundation, and National Institute of Health grant GM087413 (H Eric XU).
References
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–72. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, et al. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev. 2005;57:279–88. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- Harmar AJ. Family-B G-protein-coupled receptors. Genome Biol. 2001;2:REVIEWS3013. doi: 10.1186/gb-2001-2-12-reviews3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–57. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- Grace CR, Perrin MH, DiGruccio MR, Miller CL, Rivier JE, Vale WW, et al. NMR structure and peptide hormone binding site of the first extracellular domain of a type B1 G protein-coupled receptor. Proc Natl Acad Sci U S A. 2004;101:12836–41. doi: 10.1073/pnas.0404702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace CR, Perrin MH, Gulyas J, Digruccio MR, Cantle JP, Rivier JE, et al. Structure of the N-terminal domain of a type B1 G protein-coupled receptor in complex with a peptide ligand. Proc Natl Acad Sci U S A. 2007;104:4858–63. doi: 10.1073/pnas.0700682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthier C, Kleinschmidt M, Neumann P, Rudolph R, Manhart S, Schlenzig D, et al. Crystal structure of the incretin-bound extracellular domain of a G protein-coupled receptor. Proc Natl Acad Sci U S A. 2007;104:13942–7. doi: 10.1073/pnas.0706404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Song D, Davis-Taber RA, Barrett LW, Scott VE, Richardson PL, et al. Solution structure and mutational analysis of pituitary adenylate cyclase-activating polypeptide binding to the extracellular domain of PAC1-RS. Proc Natl Acad Sci U S A. 2007;104:7875–80. doi: 10.1073/pnas.0611397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runge S, Thogersen H, Madsen K, Lau J, Rudolph R. Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J Biol Chem. 2008;283:11340–7. doi: 10.1074/jbc.M708740200. [DOI] [PubMed] [Google Scholar]
- Pioszak AA, Xu HE. Molecular recognition of parathyroid hormone by its G protein-coupled receptor. Proc Natl Acad Sci U S A. 2008;105:5034–9. doi: 10.1073/pnas.0801027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak AA, Parker NR, Suino-Powell K, Xu HE. Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1. J Biol Chem. 2008;283:32900–12. doi: 10.1074/jbc.M805749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioszak AA, Parker NR, Gardella TJ, Xu HE. Structural basis for parathyroid hormone-related protein binding to the parathyroid hormone receptor and design of conformation-selective peptides. J Biol Chem. 2009;284:28382–91. doi: 10.1074/jbc.M109.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace CR, Perrin MH, Gulyas J, Rivier JE, Vale WW, Riek R. NMR structure of the first extracellular domain of corticotropin-releasing factor receptor 1 (ECD1-CRF-R1) complexed with a high affinity agonist. J Biol Chem. 2010;285:38580–9. doi: 10.1074/jbc.M110.121897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal K, Swaminathan K, Xu HE, Pioszak AA. Structural basis for hormone recognition by the Human CRFR2{alpha} G protein-coupled receptor. J Biol Chem. 2010;285:40351–61. doi: 10.1074/jbc.M110.186072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E, Koth CM, Abdul-Manan N, Swenson L, Coll JT, Lippke JA, et al. Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure. 2010;18:1083–93. doi: 10.1016/j.str.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Kumar S, Pioszak A, Zhang C, Swaminathan K, Xu HE. Crystal structure of the PAC1R extracellular domain unifies a consensus fold for hormone recognition by class B G-protein coupled receptors. PLoS One. 2011;6:e19682. doi: 10.1371/journal.pone.0019682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath K, Stefanatos G, Sokolski KN, Wachtel R, Nabors L, Tildon JT. Improved social and language skills after secretin administration in patients with autistic spectrum disorders. J Assoc Acad Minor Phys. 1998;9:9–15. [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Hao Z, Huang Y, Cleman J, Jovin IS, Vale WW, Bale TL, et al. Urocortin2 inhibits tumor growth via effects on vascularization and cell proliferation. Proc Natl Acad Sci U S A. 2008;105:3939–44. doi: 10.1073/pnas.0712366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole KE, Reeve J. Parathyroid hormone — a bone anabolic and catabolic agent. Curr Opin Pharmacol. 2005;5:612–7. doi: 10.1016/j.coph.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Usdin TB. The TIP39-PTH2 receptor system: unique peptidergic cell groups in the brainstem and their interactions with central regulatory mechanisms. Prog Neurobiol. 2010;90:29–59. doi: 10.1016/j.pneurobio.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y. Gastric inhibitory polypeptide (GIP) receptor. Nippon Rinsho. 1997;55:512–6. [PubMed] [Google Scholar]
- Nauck MA, Baller B, Meier JJ. Gastric inhibitory polypeptide and glucagon-like peptide-1 in the pathogenesis of type 2 diabetes. Diabetes. 2004;53:S190–6. doi: 10.2337/diabetes.53.suppl_3.s190. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. [PubMed] [Google Scholar]
- Sexton PM, Morfis M, Tilakaratne N, Hay DL, Udawela M, Christopoulos G, et al. Complexing receptor pharmacology: modulation of family B G protein-coupled receptor function by RAMPs. Ann N Y Acad Sci. 2006;1070:90–104. doi: 10.1196/annals.1317.076. [DOI] [PubMed] [Google Scholar]
- Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–6. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Dockerill S, Adamiak DA, Tickle IJ, Blundell T. X-ray analysis of glucagon and its relationship to receptor binding. Nature. 1975;257:751–7. doi: 10.1038/257751a0. [DOI] [PubMed] [Google Scholar]
- Grace CR, Perrin MH, Cantle JP, Vale WW, Rivier JE, Riek R. Common and divergent structural features of a series of corticotropin releasing factor-related peptides. J Am Chem Soc. 2007;129:16102–14. doi: 10.1021/ja0760933. [DOI] [PubMed] [Google Scholar]
- Venneti KC, Hewage CM. Conformational and molecular interaction studies of glucagon-like peptide-2 with its N-terminal extracellular receptor domain. FEBS Lett. 2011;585:346–52. doi: 10.1016/j.febslet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Runge S, Gram C, Brauner-Osborne H, Madsen K, Knudsen LB, Wulff BS. Three distinct epitopes on the extracellular face of the glucagon receptor determine specificity for the glucagon amino terminus. J Biol Chem. 2003;278:28005–10. doi: 10.1074/jbc.M301085200. [DOI] [PubMed] [Google Scholar]
- Runge S, Wulff BS, Madsen K, Brauner-Osborne H, Knudsen LB. Different domains of the glucagon and glucagon-like peptide-1 receptors provide the critical determinants of ligand selectivity. Br J Pharmacol. 2003;138:787–94. doi: 10.1038/sj.bjp.0705120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwitz C, Gardella TJ, Flannery MR. Potts JT Jr, Kronenberg HM, Goldring SR, et al. Full activation of chimeric receptors by hybrids between parathyroid hormone and calcitonin. Evidence for a common pattern of ligand-receptor interaction. J Biol Chem. 1996;271:26469–72. doi: 10.1074/jbc.271.43.26469. [DOI] [PubMed] [Google Scholar]
- Stroop SD, Kuestner RE, Serwold TF, Chen L, Moore EE. Chimeric human calcitonin and glucagon receptors reveal two dissociable calcitonin interaction sites. Biochemistry. 1995;34:1050–7. doi: 10.1021/bi00003a040. [DOI] [PubMed] [Google Scholar]
- Holtmann MH, Hadac EM, Miller LJ. Critical contributions of amino-terminal extracellular domains in agonist binding and activation of secretin and vasoactive intestinal polypeptide receptors. Studies of chimeric receptors. J Biol Chem. 1995;270:14394–8. doi: 10.1074/jbc.270.24.14394. [DOI] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Marie JC. VPAC receptors for VIP and PACAP. Receptors Channels. 2002;8:137–53. [PubMed] [Google Scholar]
- Beyermann M, Rothemund S, Heinrich N, Fechner K, Furkert J, Dathe M, et al. A role for a helical connector between two receptor binding sites of a long-chain peptide hormone. J Biol Chem. 2000;275:5702–9. doi: 10.1074/jbc.275.8.5702. [DOI] [PubMed] [Google Scholar]
- Norman DG, Barlow PN, Baron M, Day AJ, Sim RB, Campbell ID. Three-dimensional structure of a complement control protein module in solution. J Mol Biol. 1991;219:717–25. doi: 10.1016/0022-2836(91)90666-t. [DOI] [PubMed] [Google Scholar]
- Parthier C, Reedtz-Runge S, Rudolph R, Stubbs MT. Passing the baton in class B GPCRs: peptide hormone activation via helix induction. Trends Biochem Sci. 2009;34:303–10. doi: 10.1016/j.tibs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Pioszak AA, Harikumar KG, Parker NR, Miller LJ, Xu HE. Dimeric arrangement of the parathyroid hormone receptor and a structural mechanism for ligand-induced dissociation. J Biol Chem. 2010;285:12435–44. doi: 10.1074/jbc.M109.093138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. How and why do GPCRs dimerize. Trends Pharmacol Sci. 2008;29:234–40. doi: 10.1016/j.tips.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Kniazeff J, Liu J, Binet V, Goudet C, Rondard P, et al. Allosteric functioning of dimeric class C G-protein-coupled receptors. FEBS J. 2005;272:2947–55. doi: 10.1111/j.1742-4658.2005.04728.x. [DOI] [PubMed] [Google Scholar]
- Fotiadis D, Jastrzebska B, Philippsen A, Muller DJ, Palczewski K, Engel A. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr Opin Struct Biol. 2006;16:252–9. doi: 10.1016/j.sbi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Fung JJ, Deupi X, Pardo L, Yao XJ, Velez-Ruiz GA, Devree BT, et al. Ligand-regulated oligomerization of beta(2)-adrenoceptors in a model lipid bilayer. EMBO J. 2009;28:3315–28. doi: 10.1038/emboj.2009.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hern JA, Baig AH, Mashanov GI, Birdsall B, Corrie JE, Lazareno S, et al. Formation and dissociation of M1 muscarinic receptor dimers seen by total internal reflection fluorescence imaging of single molecules. Proc Natl Acad Sci U S A. 2010;107:2693–8. doi: 10.1073/pnas.0907915107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Pinon DI, Miller LJ. Transmembrane segment IV contributes a functionally important interface for oligomerization of the Class II G protein-coupled secretin receptor. J Biol Chem. 2007;282:30363–72. doi: 10.1074/jbc.M702325200. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Morfis MM, Sexton PM, Miller LJ. Pattern of intra-family hetero-oligomerization involving the G-protein-coupled secretin receptor. J Mol Neurosci. 2008;36:279–85. doi: 10.1007/s12031-008-9060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Morfis MM, Lisenbee CS, Sexton PM, Miller LJ. Constitutive formation of oligomeric complexes between family B G protein-coupled vasoactive intestinal polypeptide and secretin receptors. Mol Pharmacol. 2006;69:363–73. doi: 10.1124/mol.105.015776. [DOI] [PubMed] [Google Scholar]
- Seck T, Baron R, Horne WC. The alternatively spliced deltae13 transcript of the rabbit calcitonin receptor dimerizes with the C1a isoform and inhibits its surface expression. J Biol Chem. 2003;278:23085–93. doi: 10.1074/jbc.M211280200. [DOI] [PubMed] [Google Scholar]
- Kraetke O, Wiesner B, Eichhorst J, Furkert J, Bienert M, Beyermann M. Dimerization of corticotropin-releasing factor receptor type 1 is not coupled to ligand binding. J Recept Signal Transduct Res. 2005;25:251–76. doi: 10.1080/10799890500468838. [DOI] [PubMed] [Google Scholar]
- Maurel D, Comps-Agrar L, Brock C, Rives ML, Bourrier E, Ayoub MA, et al. Cell-surface protein-protein interaction analysis with time-resolved FRET and snap-tag technologies: application to GPCR oligomerization. Nat Methods. 2008;5:561–7. doi: 10.1038/nmeth.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesleh MF, Shirley WA, Heise CE, Ling N, Maki RA, Laura RP. NMR structural characterization of a minimal peptide antagonist bound to the extracellular domain of the corticotropin-releasing factor1 receptor. J Biol Chem. 2007;282:6338–46. doi: 10.1074/jbc.M609816200. [DOI] [PubMed] [Google Scholar]
- Inooka H, Ohtaki T, Kitahara O, Ikegami T, Endo S, Kitada C, et al. Conformation of a peptide ligand bound to its G-protein coupled receptor. Nat Struct Biol. 2001;8:161–5. doi: 10.1038/84159. [DOI] [PubMed] [Google Scholar]
- Neumann JM, Couvineau A, Murail S, Lacapere JJ, Jamin N, Laburthe M. Class-B GPCR activation: is ligand helix-capping the key. Trends Biochem Sci. 2008;33:314–9. doi: 10.1016/j.tibs.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Shimizu N, Guo J, Gardella TJ. Parathyroid hormone (PTH)-(1–14) and -(1–11) analogs conformationally constrained by alpha-aminoisobutyric acid mediate full agonist responses via the juxtamembrane region of the PTH-1 receptor. J Biol Chem. 2001;276:49003–12. doi: 10.1074/jbc.M106827200. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;98:7570–5. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin TB, Hoare SR, Wang T, Mezey E, Kowalak JA. TIP39: a new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat Neurosci. 1999;2:941–3. doi: 10.1038/14724. [DOI] [PubMed] [Google Scholar]
- Hoare SR, Clark JA, Usdin TB. Molecular determinants of tuberoinfundibular peptide of 39 residues (TIP39) selectivity for the parathyroid hormone-2 (PTH2) receptor. N-terminal truncation of TIP39 reverses PTH2 receptor/PTH1 receptor binding selectivity. J Biol Chem. 2000;275:27274–83. doi: 10.1074/jbc.M003910200. [DOI] [PubMed] [Google Scholar]
- Abraham-Nordling M, Persson B, Nordling E. Model of the complex of Parathyroid hormone-2 receptor and Tuberoinfundibular peptide of 39 residues. BMC Res Notes. 2010;3:270. doi: 10.1186/1756-0500-3-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Katsanou ES, Hillhouse EW. Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R1alpha and R2beta CRH receptor subtypes and stimulation of Gq-proteins. Mol Endocrinol. 2000;14:2076–91. doi: 10.1210/mend.14.12.0574. [DOI] [PubMed] [Google Scholar]
- Brar BK, Chen A, Perrin MH, Vale W. Specificity and regulation of extracellularly regulated kinase1/2 phosphorylation through corticotropin-releasing factor (CRF) receptors 1 and 2beta by the CRF/urocortin family of peptides. Endocrinology. 2004;145:1718–29. doi: 10.1210/en.2003-1023. [DOI] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, et al. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–64. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- Gesty-Palmer D, Flannery P, Yuan L, Corsino L, Spurney R, Lefkowitz RJ, et al. A beta-arrestin-biased agonist of the parathyroid hormone receptor (PTH1R) promotes bone formation independent of G protein activation. Sci Transl Med. 2009;1:1ra1. doi: 10.1126/scitranslmed.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare SR. Mechanisms of peptide and nonpeptide ligand binding to Class B G-protein-coupled receptors. Drug Discov Today. 2005;10:417–27. doi: 10.1016/S1359-6446(05)03370-2. [DOI] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–46. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Kusano S, Kukimoto-Niino M, Akasaka R, Toyama M, Terada T, Shirouzu M, et al. Crystal structure of the human receptor activity-modifying protein 1 extracellular domain. Protein Sci. 2008;17:1907–14. doi: 10.1110/ps.036012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangoor D, Biondi B, Gobbo M, Vachutinski Y, Fridkin M, Gozes I, et al. Novel glycosylated VIP analogs: synthesis, biological activity, and metabolic stability. J Pept Sci. 2008;14:321–8. doi: 10.1002/psc.932. [DOI] [PubMed] [Google Scholar]
- Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–9. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- Langer I, Gregoire F, Nachtergael I, De Neef P, Vertongen P, Robberecht P. Hexanoylation of a VPAC2 receptor-preferring ligand markedly increased its selectivity and potency. Peptides. 2004;25:275–8. doi: 10.1016/j.peptides.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Xiao Q, Giguere J, Parisien M, Jeng W, St-Pierre SA, Brubaker PL, et al. Biological activities of glucagon-like peptide-1 analogues in vitro and in vivo. Biochemistry. 2001;40:2860–9. doi: 10.1021/bi0014498. [DOI] [PubMed] [Google Scholar]
- Spyroulias GA, Papazacharias S, Pairas G, Cordopatis P. Monitoring the structural consequences of Phe12 → D-Phe and Leu15 → Aib substitution in human/rat corticotropin releasing hormone. Implications for design of CRH antagonists. Eur J Biochem. 2002;269:6009–19. doi: 10.1046/j.1432-1033.2002.03278.x. [DOI] [PubMed] [Google Scholar]
- Gault VA, Kerr BD, Irwin N, Flatt PR. C-terminal mini-PEGylation of glucose-dependent insulinotropic polypeptide exhibits metabolic stability and improved glucose homeostasis in dietary-induced diabetes. Biochem Pharmacol. 2008;75:2325–33. doi: 10.1016/j.bcp.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Gallwitz B. The evolving place of incretin–based therapies in type 2 diabetes. Pediatr Nephrol. 2010;25:1207–17. doi: 10.1007/s00467-009-1435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichman SL, Neale A, Lawrence B, Gagnon C, Castaigne JP, Frohman LA. Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. J Clin Endocrinol Metab. 2006;91:799–805. doi: 10.1210/jc.2005-1536. [DOI] [PubMed] [Google Scholar]
- Chunxiao W, Jingjing L, Yire X, Min D, Zhaohui W, Gaofu Q, et al. Study on preparation and activity of a novel recombinant human parathyroid hormone(1–34) analog with N-terminal Pro-Pro extension. Regul Pept. 2007;141:35–43. doi: 10.1016/j.regpep.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Underwood CR, Garibay P, Knudsen LB, Hastrup S, Peters GH, Rudolph R, et al. Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J Biol Chem. 2010;285:723–30. doi: 10.1074/jbc.M109.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]