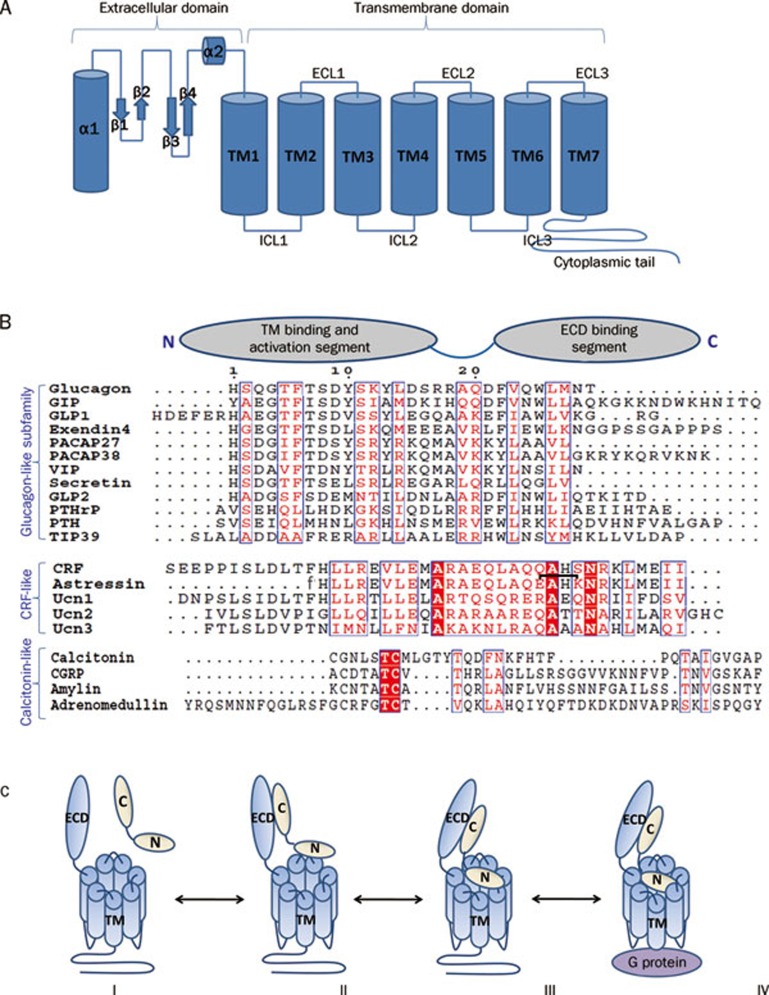
Figure 1.
(A) Cartoon presentation of the general architecture of Class B GPCRs consisting of a N-terminal extracellular domain (ECD) and a C-terminal transmembrane domain (7TM). The ECD forms a three layer α-β-β/α fold and the 7TM domain seven membrane-spanning helices connected by three extracellular loops (ECLs) and three intracellular loops (ICLs). (B) Sequence alignment of Class B GPCR ligands with cartoon presentation of their N- and C-terminal domains on top. Based on sequence similarity, ligands can be grouped into glucagon-like, CRF-like, and calcitonin-like subfamilies. Identical residues are shown as white letters on red background. Partially conserved residues are shown as red letters. The residue numbering on top corresponds to that of glucagon. The lactam bridge in astressin is indicated by a black bracket, “f” in the astressin sequence indicates D-phenylalanine. (C) Two domain binding model for class B GPCRs. (I) Peptide hormone and receptor are orientated for initial receptor ligand binding. (II) The initial complex forms between the C-terminus of the peptide and the ECD of the receptor. (III) This interaction facilitates the binding of the free N-terminus of the peptide to the juxtamembrane region of the 7TM domain of the receptor. (IV) This binding induces a conformational change in the 7TM and cytoplasmic domain of the receptor, which mediates its interaction with a heterotrimeric G protein.