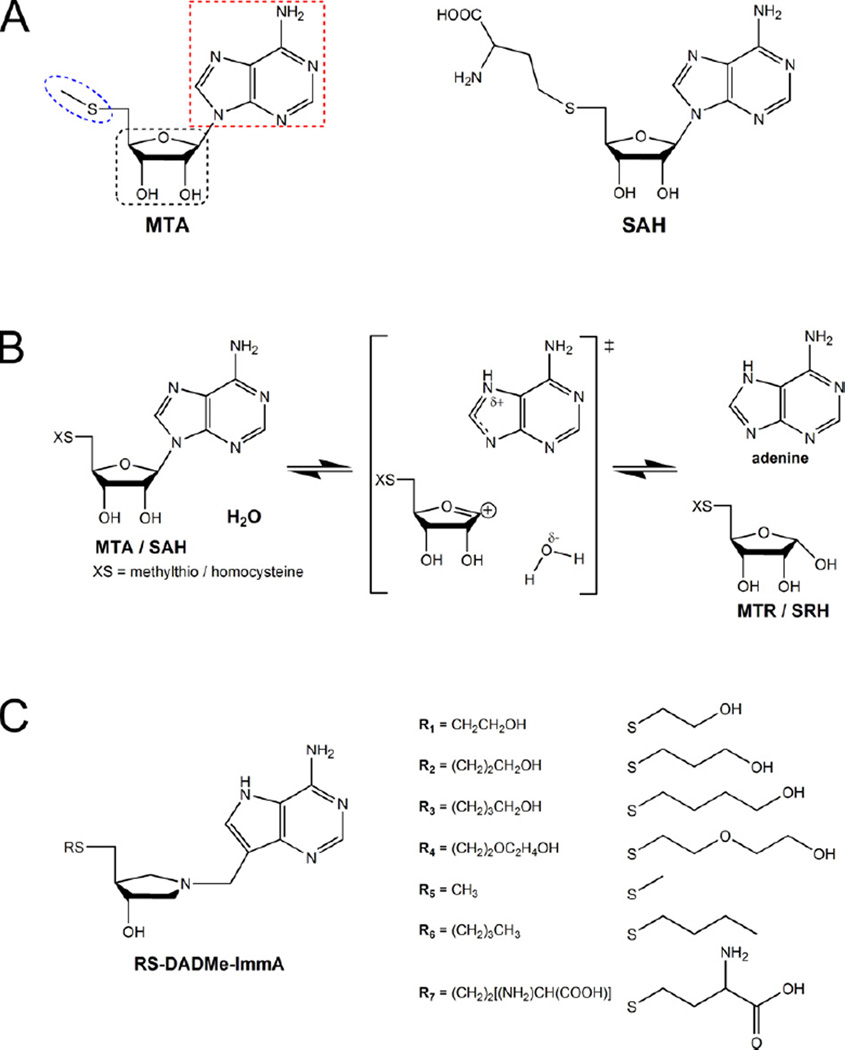
Figure 3.
The substrates and transition state analogues of S. enterica MTAN (SeMTAN). (A) Methylthioadenosine (MTA) and S-adenosylhomocysteine (SAH) are the substrates of MTAN. The substrates and substrate analogues of MTAN can be divided into adenine (red dotted line), ribose (black dotted line) and 5’-alkylthio (blue dotted line) moieties. All these moieties have defined subsites in the active site of MTAN (Lee et al., 2003). (B) The reaction as catalyzed by MTAN. The transition state of MTAN involves a dissociated adenine leaving group and ribocation. In the end of the reaction cycle, the carbocation is neutralized by the activated water molecule producing either methylthioribose (MTR) of S-ribosylhomocysteine (SRH) together with adenine. (C) Transition state analogues. The DADMe-ImmA inhibitors mimic the late dissociative transition state character of MTAN, in which the distance between C1´ of thioribose and N9 of adenine is approximately 3 Å, and they have two chiral centers (3R,4S). The RS-DADMe-ImmA having R1–R4 groups are novel inhibitors designed on the SeMTAN crystal structure in complex with TEG (see also Figure S1). R5 is the transition state mimic of MTA and R7 is the transition state mimic of SAH.