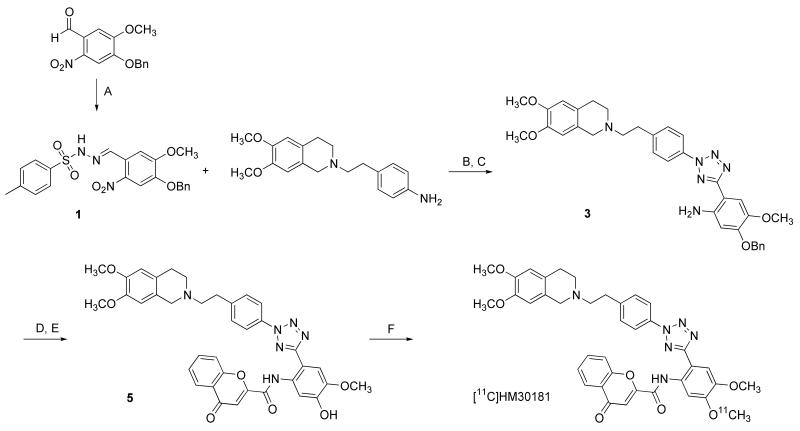
Fig. 1.
Synthesis of O-desmethyl-HM30181 (5) and [11C]HM30181 by [11C]methylation with [11C]methyl triflate. Reagents: (A) 4-methylbenzenesulfonohydrazide, ethanol, reflux; (B) NaNO2, aq. HCl, ethanol, pyridine, 0°C to −15°C; (C) Raney-Nickel, (NH2)2·H2O, tetrahydrofuran/ethanol, reflux; (D) 4-oxo-4H-chromene-2-carboxylic acid, HOBt, EDC·HCl, DMF, 0°C to room temperature; (E) trifluoroacetic acid, thioanisole, room temperature; (F) [11C]methyl triflate, TBAH, DMF/acetonitrile, 75°C.