SUMMARY
In animals, both siRNAs and miRNAs are thought to diminish protein synthesis from transcripts that are perfectly complementary by directing endonucleolytic cleavage where they anneal, thereby triggering rapid degradation of the entire message [1–4]. By contrast, partially complementary messages are downregulated by a combination of translational repression and accelerated decay caused by rapid poly(A) tail removal [3, 5–12]. Here we present evidence that translational repression can also make a substantial contribution to the downregulation of fully complementary messages by RNA interference. Unlike mRNA destabilization, this inhibitory effect on translation is greater for perfectly complementary elements located in the 3′ untranslated region rather than in the protein-coding region. In addition to known disparities in their endonucleolytic activity [13, 14], the four Ago proteins with which siRNAs associate in humans differ significantly in their capacity to direct translational repression. As a result, the relative effect of siRNA on targets that are fully versus partially complementary is influenced by the comparative abundance of the three non-nucleolytic Ago proteins, causing this on-target/off-target ratio to vary in a cell-type-dependent manner due to the dissimilar tissue distribution of these proteins. These findings have important implications for the efficacy and specificity of RNA interference.
RESULTS
Contribution of translational repression to on-target RNA interference
Despite their dissimilar biogenesis, there appear to be no intrinsic differences in the means by which small interfering RNAs (siRNAs) and microRNAs (miRNAs) can inhibit gene expression in animals [2, 3, 7]. For both, the mechanism of repression depends on their degree of complementarity to the messages they target. The regulatory influence of siRNAs and miRNAs is a consequence of their association with a multimeric assembly known as RISC [15]. The RISC subunit to which the si/miRNA binds is the Ago protein, of which there are four in humans [15]. Unlike the two more specialized Ago proteins of Drosophila [16, 17], all four human proteins associate with both siRNA and miRNA [13, 14]. However, only one of them (Ago2) functions as an endonuclease that can cleave mRNA molecules within regions that base pair with perfectly complementary siRNAs or miRNAs [13, 14]. The presence of three other, catalytically inactive Ago proteins capable of delivering siRNAs to their targets (Ago1, Ago3, and Ago4) raised the possibility that a non-nucleolytic mechanism might also contribute to RNA interference (RNAi) by fully complementary siRNAs in mammalian cells.
To address this question, 293T cells were transiently cotransfected with a chemically synthesized siRNA (siEGFP) and a firefly luciferase (FL) reporter bearing either a single copy of a perfectly complementary element (GP) or 1–4 copies of a partially complementary element (GM) within the 3′ untranslated region (UTR) (Figure 1A). The effects of the siRNA on mRNA decay and translation efficiency (protein synthesis per mRNA molecule) were then determined by comparing the cellular concentration of the reporter mRNA and its protein product in the presence or absence of siEGFP. For each element, both accelerated mRNA decay and translational repression were found to contribute significantly to reporter downregulation (Table 1; Supplementary Figure S1). Thus, interaction of siEGFP with the perfectly complementary element GP not only directed endonucleolytic cleavage, thereby diminishing the cytoplasmic concentration of uncleaved FL+GP mRNA by a factor of 3.4 ± 0.3, but also decreased its translation efficiency by a factor of 3.4 ± 0.4, resulting in a 91% (factor of 11.4 ± 1.0) overall reduction in luciferase synthesis. These effects were entirely dependent on the elements GP and GM, as evidenced by the inability of siEGFP to influence an otherwise identical reporter transcript lacking either element. The decrease in translation efficiency attributable to a single copy of the fully complementary element GP (factor of 3.4 ± 0.4) was significantly greater than that caused by one copy of the imperfectly complementary element GM (factor of 1.7 ± 0.1).
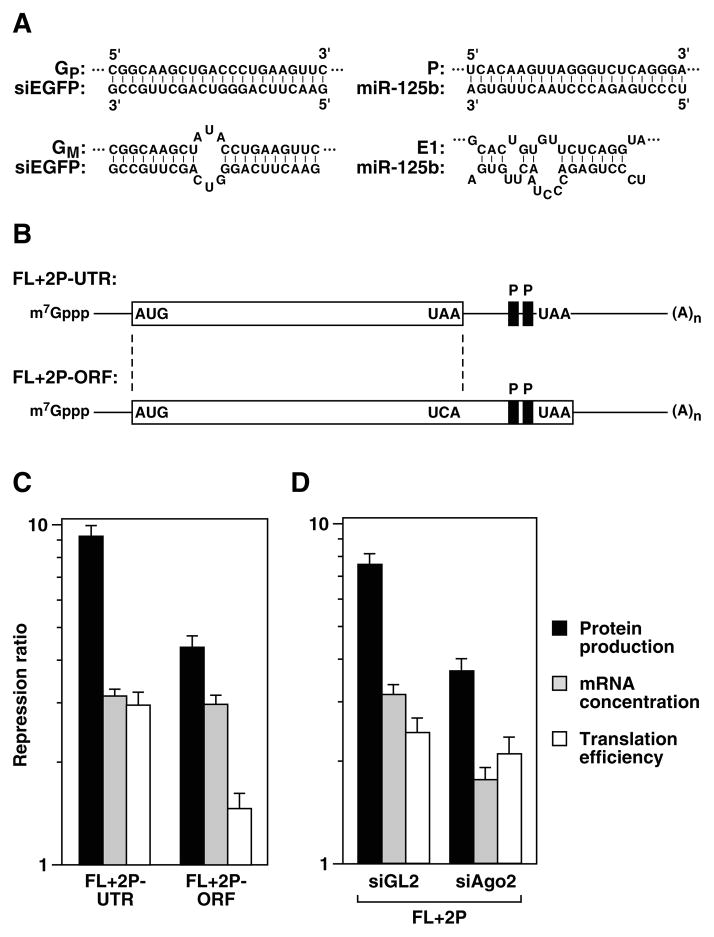
Figure 1. Effect of miR-125b and siEGFP on reporter mRNA abundance.
(A) RNA duplexes expected for siEGFP base paired with element GP or GM and for miR-125b base paired with element P or the E1 element of human lin-28.
(B) FL+2P-UTR and FL+2P-ORF mRNA. These messages were identical except for a codon substitution (UAA → UCA) that extended the protein-coding region of FL+2P-ORF beyond the two copies of element P (black rectangles). White rectangles, coding regions; lines, untranslated regions.
(C) Effect of ribosomal readthrough on the contributions of translational repression and diminished mRNA concentration to RNA interference. 293T cells were transiently cotransfected with an FL+2P-UTR or FL+2P-ORF reporter gene, a gene encoding or not encoding miR-125b, and a Renilla luciferase (RL) gene (internal standard). Repression ratios were measured as in Table 1. miR-125b had no effect on similar reporters that lacked element P.
(D) Effect of Ago2 deficiency on the contributions of translational repression and diminished mRNA concentration to RNA interference. 293T cells were transiently transfected with siRNA complementary to Ago2 mRNA, resulting in a 73% reduction in the cellular concentration of Ago2 protein, or with GL2 siRNA (negative control), and effects on the downregulation of FL+2P by miR-125b were measured.
Table 1. Contributions of impaired translation and diminished mRNA concentration to repression by perfectly or imperfectly complementary siRNA and miRNA.
The effect of siEGFP or miR-125b on reporter gene expression was determined at both the protein and mRNA level by measuring firefly luciferase activity (normalized to Renilla luciferase activity) and by Northern blot analysis of the firefly luciferase reporter transcript (normalized to Renilla luciferase mRNA) (Supplementary Figure 1) 36 hr after transfection of 293T cells. Repression ratios for protein production and cytoplasmic mRNA concentration were calculated from normalized levels of firefly luciferase protein and uncleaved mRNA in the absence versus the presence of siEGFP or miR-125b. By dividing these two repression ratios, the repression ratio for translation efficiency (protein yield per mRNA molecule) was determined. A repression ratio of 1 indicates no repression. Calculations of repression ratio per element assume that the fold effects of all copies of an element are equal and multiplicative, as observed for E1 and miR-125b [10].
| Repression ratio | |||||||
|---|---|---|---|---|---|---|---|
| 3′ UTR element | Copies | mi/siRNA | Protein production | mRNA concentration | Translation efficiency | ||
| Total | Per element | Total | Per element | ||||
| None | 0 | siEGFP | 00.98 ± 0.05 | 1.00 ± 0.06 | 0.98 ± 0.08 | ||
| GP | 1 | siEGFP | 11.43 ± 0.96 | 3.37 ± 0.26 | 3.37 ± 0.26 | 3.39 ± 0.38 | 3.39 ± 0.38 |
| GM | 1 | siEGFP | 02.79 ± 0.17 | 1.60 ± 0.08 | 1.60 ± 0.08 | 1.74 ± 0.14 | 1.74 ± 0.14 |
| GM | 4 | siEGFP | 10.83 ± 0.71 | 2.27 ± 0.20 | 1.23 ± 0.03 | 4.76 ± 0.52 | 1.48 ± 0.04 |
| None | 0 | miR-125b | 01.02 ± 0.05 | 1.00 ± 0.05 | 1.02 ± 0.07 | ||
| P | 2 | miR-125b | 08.07 ± 0.39 | 3.10 ± 0.22 | 1.76 ± 0.06 | 2.60 ± 0.22 | 1.61 ± 0.07 |
| E1 | 2 | miR-125b | 02.02 ± 0.19 | 1.39 ± 0.06 | 1.18 ± 0.03 | 1.45 ± 0.15 | 1.20 ± 0.06 |
| E1 | 6 | miR-125b | 09.09 ± 0.44 | 2.81 ± 0.14 | 1.19 ± 0.01 | 3.23 ± 0.22 | 1.22 ± 0.02 |
Translational repression also contributes to downregulation by fully complementary miRNAs transcribed in the nucleus and exported to the cytoplasm. This conclusion was drawn from experiments in which 293T cells were transiently cotransfected with a gene encoding (or not encoding) miR-125b and a firefly luciferase reporter whose 3′ UTR bore either two copies of a synthetic element (P) to which that microRNA was perfectly complementary or 2–6 copies of the imperfectly complementary E1 element of human lin-28 (Figure 1A). Once again, significant reductions in both mRNA abundance and translation efficiency were observed in each case, whereas no such effects were evident for a similar reporter lacking these elements (Table 1; Supplementary Figure S1). Moreover, as seen for siEGFP, the reduction in translation efficiency caused by two copies of the perfectly complementary element P (factor of 2.6 ± 0.2) was significantly greater than that caused by two copies of the imperfectly complementary element E1 (factor of 1.5 ± 0.2) or other well-matched elements with incomplete complementarity to miR-125b [10]. That similar results were obtained with siEGFP and miR-125b 36 or 60 hr post-transfection (Table 1 and Supplementary Table S1) indicates that sufficient time elapsed for reporter mRNA and protein levels to adjust fully. We conclude that translational repression can make an important contribution to the regulatory influence of perfectly complementary siRNAs and miRNAs, regardless of their origin or how they reach the cytoplasm.
A role for translational repression in RNA interference by fully complementary si/miRNAs in animal cells has not previously been noted. Indeed, prior evidence that mouse embryo fibroblasts lacking an Ago2 gene are competent for repression by imperfectly complementary siRNA but not by perfectly complementary siRNA [14] had cast doubt on a contribution from non-nucleolytic Ago proteins to downregulation by fully complementary siRNAs. One difference between those earlier studies and our own was the location of the fully complementary elements in either the coding region or 3′ UTR, respectively, of the reporter mRNAs. To determine whether the location of such perfectly complementary elements influences the degree to which they inhibit translation, we compared the contributions of translational repression and accelerated mRNA decay to miR-125b-mediated downregulation of two very similar reporters (FL+2P-UTR and FL+2P-ORF) bearing tandem copies of element P in virtually identical sequence contexts but distinct translational contexts. These reporters differed only by a single nucleotide substitution in FL+2P-ORF that inactivated the usual luciferase termination codon (UAA → UCA) and extended the translational open reading frame beyond the two copies of element P located 69–124 nt downstream (Figure 1B). miR-125b had a comparable effect on the stability of both messages; however, its inhibitory effect on translation, which was quite pronounced when the copies of element P were in the 3′ UTR (FL+2P-UTR), was much smaller when they were in the coding region (FL+2P-ORF) (Figure 1C). We conclude that the contribution of translational repression to RNA interference by perfectly complementary si/miRNAs depends upon whether they anneal within a translated or untranslated region of a message.
As Ago2 is the only Ago protein in humans that can cleave mRNA endonucleolytically in the presence of a fully complementary siRNA or miRNA, we investigated the degree to which reducing its abundance impairs RNA interference. Knocking down the concentration of Ago2 in 293T cells significantly decreased the ability of miR-125b to direct endonucleolytic cleavage of a reporter mRNA bearing 3′ UTR elements to which it was perfectly complementary, as evidenced by the increased abundance of intact FL+2P mRNA and the reduced concentration of the expected 5′ cleavage product (Figure 1D; Supplementary Figure S2). On the other hand, this change in the level of Ago2 had a negligible impact on the ability of miR-125b to downregulate the translation efficiency of that reporter (Figure 1D), suggesting an important role for the other three Ago proteins (see below). Consistent with this conclusion, knocking down Ago2 had a significantly greater effect on miR-125b repression of FL+2P-ORF, whose downregulation depends primarily on endonucleolytic cleavage by Ago2, than FL+2P-UTR, whose downregulation involves significant contributions from both translational repression and accelerated mRNA decay, likely mediated in part by other Ago proteins (Supplementary Figure S3).
Differences in translational repression potency among Ago proteins
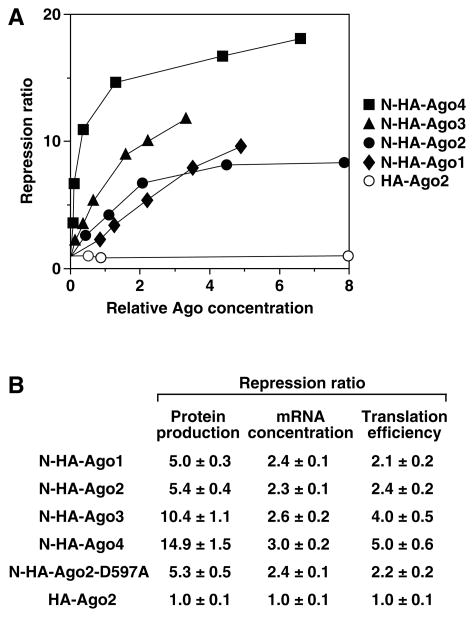
Previous studies have shown that Ago2, Ago3, and Ago4 are each able to downregulate gene expression in an si/miRNA-independent manner when tethered to a reporter mRNA via a heterologous RNA-binding domain to which they are fused [18]. However, the relative efficacy of the four human paralogs and the contributions of translational repression and accelerated mRNA decay to their overall effect remained unclear, as significant disparities in the degree of repression caused by tethering Ago2 have been reported by different laboratories [18–20]. To quantify the ability of all four human Ago proteins to downregulate gene expression and to determine the mechanism(s) by which they do so, we tethered each of them individually to the 3′ UTR of a luciferase reporter mRNA via a fused RNA-binding domain derived from the N protein of bacteriophage lambda and the RNA ligand of that domain (the lambda boxB stem-loop) [21]. To improve the sensitivity and precision of the measurements, we used a Renilla luciferase reporter bearing ten copies of boxB (RL+10boxB) [19, 20].
293T cells were transiently cotransfected with the RL+10boxB reporter and various amounts of a gene encoding the 22-amino-acid boxB-binding domain of N fused to HA-tagged Ago1, Ago2, Ago3, or Ago4 (N-HA-Ago). The efficacy of the human Ago proteins in repressing gene expression was compared over a broad concentration range by assaying cell extracts for luciferase activity and probing immunoblots for N-HA-Ago. Interestingly, while all four tethered Ago proteins were able to downregulate reporter expression, their efficacies differed significantly, in the order Ago4 > Ago3 > Ago2 ≥ Ago1 (Figure 2A). At low concentrations, where the differences were greatest, Ago4, Ago3, and Ago2 were about 6-fold, 2.7-fold, and 1.6-fold more potent than Ago1, respectively. As expected, repression in the absence of a complementary si/miRNA required tethering and was abolished by removing the boxB-binding domain (HA-Ago2), but it did not require endonucleolytic activity, as evidenced by mutating a critical active-site residue of Ago2 (N-HA-Ago2-D597A) [14].
Figure 2. Effects of tethered Ago proteins on translation and mRNA concentration.
(A) Dose dependence of repression by tethered Ago proteins. 293T cells were transiently cotransfected with a Renilla luciferase reporter gene bearing 10 copies of boxB, various amounts of a gene encoding an HA-tagged human Ago protein fused (or not fused) to a boxB-binding domain, and a firefly luciferase gene (internal standard). Repression ratios were calculated by comparing Renilla luciferase activity in the presence or absence of each HA-tagged Ago protein and plotted as a function of the relative concentration of that protein, as determined by immunoblot analysis with anti-HA antibodies (Supplementary Figure S4). As expected, tethering a nonfunctional Ago2-F470V/F505V mutant [22] had no inhibitory effect (data not shown).
(B) Contributions of impaired translation and diminished mRNA concentration to repression by tethered Ago proteins. 293T cells were transiently transfected as in (A), with adjustments to equalize production of the various HA-tagged Ago proteins. The effect of each Ago paralog on reporter gene expression was determined at the protein level as in (A) and at the mRNA level by Northern blot analysis, and repression ratios were calculated from these values. A repression ratio of one (as observed for untethered HA-Ago2 lacking a boxB-binding domain) indicates no repression.
Contrary to earlier reports [18, 22], the tethered Ago proteins not only repressed gene expression but also mimicked the ability of microRNAs to accelerate mRNA decay, as evidenced by the diminished abundance of the luciferase reporter transcript in each case (Figure 2B, Supplementary Figure S5). Interestingly, despite substantial differences in their overall potency, the four tethered proteins caused nearly equal reductions in cytoplasmic mRNA concentration, suggesting that each has a similar capacity to destabilize mRNA. This finding indicates that the marked disparities between the effects of the tethered Ago proteins on gene expression result almost entirely from significant differences in the ability of each to repress translation (Figure 2B).
Cell-type-dependent specificity of RNA interference
Among the complications of using RNA interference to knock down gene expression is the detriment to specificity caused by off-target effects, which appear to result from base pairing of siRNAs with messages to which they are partially complementary in a manner reminiscent of productive base pairing by miRNAs [23]. That the four Ago proteins in humans differ not only in their endonucleolytic activity but also in their capacity to repress translation raised the possibility that variations in the relative abundance of these or other proteins might cause the specificity of RNA interference to be cell-type-dependent. Indeed, a previous study indicated that the human Ago paralogs are differentially expressed in several immortalized cell lines [13].
To investigate the expression of the various Ago proteins in the untransformed cells of primary tissues, we extracted data from a large-scale study of the human transcriptome [24]. Those microarray data indicate that each of the four Ago genes undergoes significant transcription in all 73 untransformed human tissues that were tested. Importantly, our analysis reveals a great deal of paralog-specific variation in Ago mRNA levels across a range of tissue and cell types (Supplementary Figure S6): up to 3- or 4-fold for Ago1, Ago3, and Ago4 and up to 6-fold for Ago2, whose abundance relative to each of the other three Ago mRNAs can vary by as much as a factor of 10.
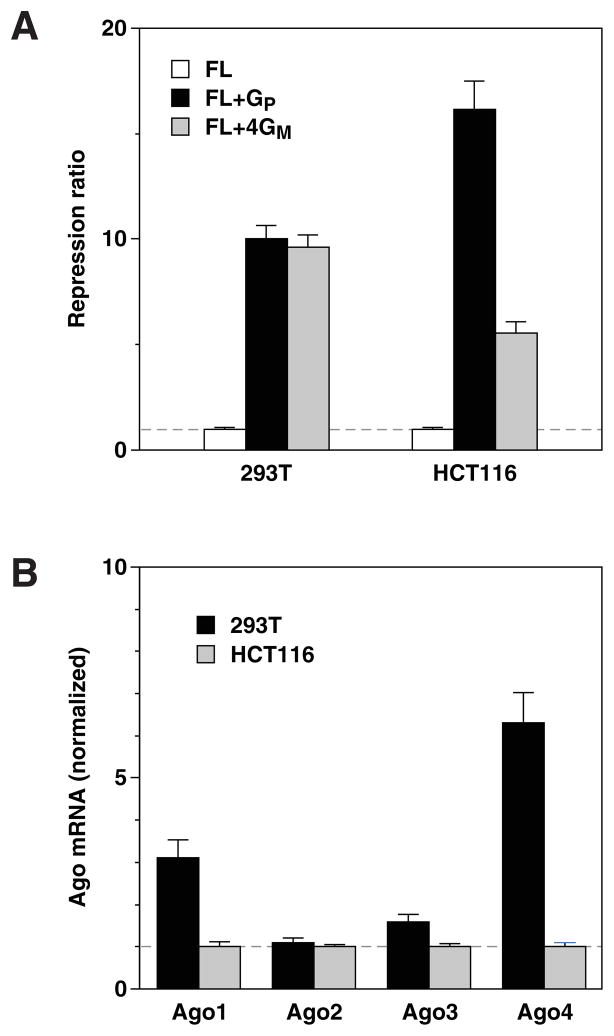
Such differences have the potential to influence the specificity of RNA interference in a cell-type-dependent manner. As a preliminary test of this hypothesis, we first compared the efficacy of siEGFP in downregulating the expression of fully (FL+GP) or partially (FL+4GM) complementary reporter genes in two human cell lines: 293T embryonic kidney cells and HCT116 colon cancer cells. Interestingly, the specificity of siEGFP for the reporter to which it was perfectly complementary was significantly greater in HCT116 cells (Figure 3A), a difference consistent with the lower concentration of Ago1, Ago3, and Ago4 mRNA and equivalent concentration of Ago2 mRNA in those cells versus 293T cells (Figure 3B; Supplementary Figure S7). A similar inverse correlation was observed in HeLa cervical carcinoma cells (Supplementary Figure S8).
Figure 3. Differential specificity of RNA interference in human cell lines.
(A) Specificity of RNA interference. The ability of siEGFP to repress luciferase production from reporter genes containing no complementary elements (FL), one perfectly complementary element (FL+GP) or four imperfectly complementary elements (FL+4GM) was compared in 293T cells and HCT116 cells.
(B) Relative concentration of Ago mRNAs. Quantitative RT-PCR was used to compare the concentrations of messages encoding each of the four Ago proteins in 293T cells and HCT116 cells. The abundance of each transcript was normalized to its level in HCT116 cells. The greater abundance of Ago1 and similar abundance of Ago2 in 293T versus HCT116 cells was confirmed by immunoblot analysis with monoclonal antibodies (Supplementary Figure S7). Antibodies that can specifically detect Ago3 and Ago4 in cell extracts are not yet available.
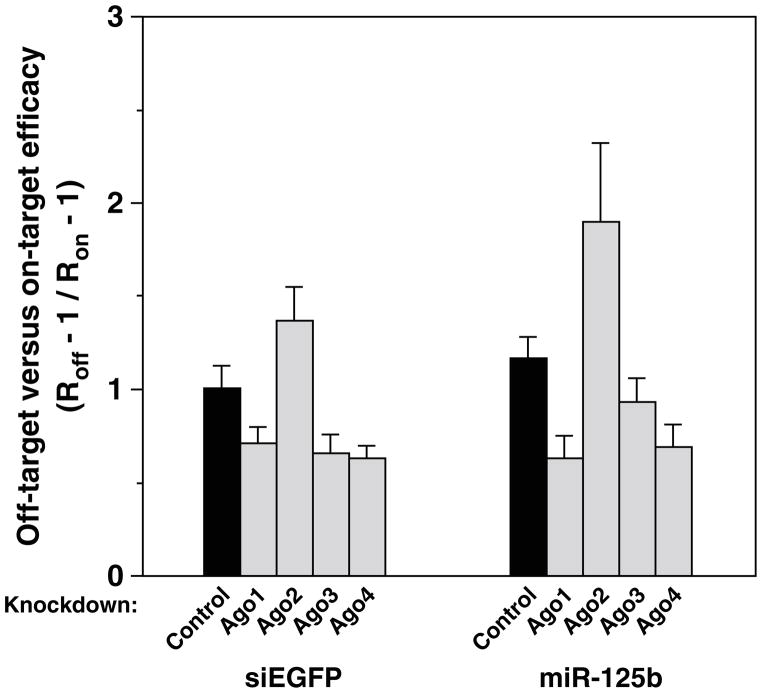
To ascertain directly whether the relatively high abundance of the non-nucleolytic Ago proteins in 293T cells impairs the specificity of RNA interference, we examined the consequences of reducing their concentration or that of Ago2. Depleting Ago1, Ago3, or Ago4 diminished the relative efficacy with which siEGFP repressed the imperfectly complementary reporter FL+4GM versus its perfectly complementary counterpart FL+GP, whereas depleting Ago2 had the opposite effect (Figure 4; Supplementary Figure S9). Similar changes in off-target versus on-target repression were observed for miR-125b and the reporters FL+2P and FL+6E1 (Figure 4). These results indicate that the specificity of RNA interference depends, at least in part, on the concentration of the three non-nucleolytic Ago proteins relative to Ago2.
Figure 4. Influence of non-nucleolytic Ago proteins on the specificity of RNA interference.
The ability of siEGFP and miR-125b to repress luciferase production from reporter genes containing elements that were perfectly (on-target: FL+GP or FL+2P) or imperfectly (off-target: FL+4GM or FL+6E1) complementary was compared in 293T cells from which each of the four Ago proteins had been depleted individually by RNAi. siGL2 served as a negative control (black bars). Specificities of RNA interference were compared by dividing the off-target and on-target repression ratios (Roff and Ron) after first subtracting 1 from each, because a repression ratio of 1 indicates a complete lack of downregulation. As judged by quantitative RT-PCR, the knockdown efficiency of the targeted Ago mRNAs was 0 ± 5% for siGL2, 86 ± 5% for siAgo1, 79 ± 5% for siAgo2, 73 ± 3% for siAgo3, and 81 ± 4% for siAgo4. As judged by immunoblotting, the knockdown efficiency was 91% for siAgo1 and 85% for siAgo2 (Supplementary Figure S9).
DISCUSSION
Together, these findings impact our understanding of RNA interference in three ways. First, they redefine the mechanism by which RNAi inhibits mammalian gene expression by revealing an important contribution from impaired translation. Second, they indicate that the magnitude of this effect depends on where in a message siRNA binding occurs and which Ago paralog accompanies it there. Third, they show that the specificity with which RNA interference targets mammalian mRNAs containing a fully complementary sequence element is cell-type-dependent and that this property is influenced, at least in part, by natural variations in the relative abundance of nucleolytic versus non-nucleolytic Ago proteins.
Initially, the complementarity of miRNAs and siRNAs to their mRNA targets was thought to determine whether gene expression would be downregulated via translational repression or instead via accelerated mRNA decay triggered by endonucleolytic cleavage [2, 3, 7]. Subsequently, it became clear that in animals the interaction of these small RNAs with messages to which they are imperfectly complementary leads both to inhibited translation and to expedited mRNA decay caused by rapid deadenylation [8–12]. Our present results extend this mechanistic duality to the interaction of siRNAs and miRNAs with messages to which they are fully complementary by showing that in mammalian cells such base pairing can result not only in endonucleolytic cleavage but also in a significant decrease in the efficiency with which those mRNAs are translated, especially when the si/miRNA anneals to the 3′ untranslated region. As a result, the overall influence of siRNAs and miRNAs on the expression of genes that are fully complementary can be substantially greater than their effect on mRNA concentration alone. This finding implies that changes in mRNA levels may understate both the specificity and impact of RNA interference. Although the inhibitory effect on translation is significantly smaller when an siRNA binds to a perfectly complementary site within a coding region, targeting the coding region can nevertheless be as effective overall as targeting the 3′ UTR due to other, counteracting influences.
That the place where a fully complementary si/miRNA binds should influence its effect on translation but not mRNA degradation is probably due to the fact that only the former repression mechanism is reversible. Consequently, an siRNA must remain bound to a message for even the leaky translational repression characteristic of RISC to persist. Such continuity is more likely if the siRNA binds to the 3′ UTR rather than to the coding region, where the occasional passage of translating ribosomes would displace it. In contrast, if endonucleolytic cleavage by Ago2 is swift, a fully complementary si/miRNA would need to bind a message only transiently to irreversibly trigger its decay, making that outcome less susceptible to disruption by ribosomes.
While translational repression by perfectly complementary siRNAs or miRNAs in animal cells has not previously been noted, it is sometimes evident upon detailed re-examination of published data in which the 3′ UTR was targeted (see, for example, the data of [4]). In other cases, such an effect may have been overlooked for any number of reasons: targeting of the coding region, insufficiently precise measurements of mRNA and protein concentrations, inadequate time for full adjustment of the protein concentration, uncertainty about possible contributions from additional, partially complementary elements within the same message, etc. It is noteworthy that the converse ability of miRNAs to accelerate the degradation of imperfectly complementary mRNAs was likewise not recognized initially but is now well established [8–12].
Translational repression by fully complementary siRNAs makes sense mechanistically, as all four Ago proteins in human cells can inhibit translation when tethered to mRNA but only Ago2 can cleave RNA endonucleolytically. Thus, every interaction of an siRNA with the 3′ UTR of a fully complementary message has the potential to be productive, even if Ago2 does not participate.
Our tethering data further indicate that the four Ago proteins in humans (possibly in conjunction with other RISC components) differ significantly in the efficacy with which they repress translation. Together with their variable tissue distribution, these differences suggest that the overall effectiveness of siRNAs and miRNAs in downregulating gene expression is likely to be cell-type-dependent.
The specificity of RNA interference is limited by the potential for off-target effects caused by the unwanted interaction of siRNAs with messages to which they are partially complementary [23]. The ability of Ago1, Ago3, and Ago4 to downregulate such off-targets without contributing to the endonucleolytic cleavage of fully complementary messages suggests that RNAi may be least specific in human tissues that produce high levels of these three proteins, a prediction corroborated by examining three human cell lines (293T, HeLa, and HCT116). Indeed, depleting 293T cells of Ago1, Ago3, or Ago4 increases the specificity of RNA interference, whereas depleting Ago2 diminishes that specificity. These findings indicate that the concentration of the non-nucleolytic paralogs is an important factor contributing to the relative magnitude of off-target repression and its cell-type dependence in humans.
Supplementary Material
Acknowledgments
We are grateful to Gunter Meister for generously providing monoclonal antibodies specific for Ago1 and Ago2. These studies were supported by research grants to J. G. B. from the National Institutes of Health (GM55624 and GM79477) and a postdoctoral fellowship to L. W. from the Vilcek Endowment.
Footnotes
EXPERIMENTAL PROCEDURES
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 2.Hutvágner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 3.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 5.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 6.Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- 7.Doench JG, Petersen CP, Sharp PA. siRNAs can function as miRNAs. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- 9.Bagga S, Bracht J, Hunter S, Massirer K, Holtz J, Eachus R, Pasquinelli AE. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell. 2005;122:553–563. doi: 10.1016/j.cell.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 10.Wu L, Belasco JG. Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells. Mol Cell Biol. 2005;25:9198–9208. doi: 10.1128/MCB.25.21.9198-9208.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA. 2006;103:4034–4039. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Van Dongen S, Inoue K, Enright AJ, Schier AF. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science. 2006;312:75–79. doi: 10.1126/science.1122689. [DOI] [PubMed] [Google Scholar]
- 13.Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 15.Peters L, Meister G. Argonaute proteins: mediators of RNA silencing. Mol Cell. 2007;26:611–623. doi: 10.1016/j.molcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Okamura K, Ishizuka A, Siomi H, Siomi MC. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–1666. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Förstemann K, Horwich MD, Wee L, Tomari Y, Zamore PD. Drosophila microRNAs are sorted into functionally distinct Argonaute complexes after production by Dicer-1. Cell. 2007;130:287–297. doi: 10.1016/j.cell.2007.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pillai RS, Artus CG, Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10:1518–1525. doi: 10.1261/rna.7131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Rivas FV, Wohlschlegel J, Yates JR, 3rd, Parker R, Hannon GJ. A role for the P-body component GW182 in microRNA function. Nat Cell Biol. 2005;7:1261–1266. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasudevan S, Steitz JA. AU-rich-element-mediated upregulation of translation by FXR1 and Argonaute 2. Cell. 2007;128:1105–1118. doi: 10.1016/j.cell.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baron-Benhamou J, Gehring NH, Kulozik AE, Hentze MW. Using the λN peptide to tether proteins to RNAs. Methods Mol Biol. 2004;257:135–154. doi: 10.1385/1-59259-750-5:135. [DOI] [PubMed] [Google Scholar]
- 22.Kiriakidou M, Tan GS, Lamprinaki S, De Planell-Saguer M, Nelson PT, Mourelatos Z. An mRNA m7G cap binding-like motif within human Ago2 represses translation. Cell. 2007;129:1141–1151. doi: 10.1016/j.cell.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, Li B, Cavet G, Linsley PS. Expression profiling reveals off-target gene regulation by RNAi. Nat Biotechnol. 2003;21:635–637. doi: 10.1038/nbt831. [DOI] [PubMed] [Google Scholar]
- 24.Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, Cooke MP, Walker JR, Hogenesch JB. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci USA. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.