Abstract
Chronic hepatitis C virus (HCV) infection affects some 170 million people worldwide, including 3 to 4 million in the United States who are largely unaware of their infection status. HCV has 6 genotypes; genotype 1 is the most common in the United States and genotypes 1 and 4 are less responsive to interferon alfa-based therapy than the other genotypes. Treatment with available direct-acting antiviral (DAA) drugs has increased sustained virologic response (SVR) rates in genotype 1 infection and shortened duration of therapy in many patients, but at this time these agents must still be administered with peginterferon alfa and ribavirin to prevent rapid emergence of resistance. Baseline predictors of response to therapy continue to play a role with triple-drug combination therapy including the pharmacogenetic IL28B genotype, which differs in prevalence throughout the world. The stopping/futility rules are different for triple-drug combination therapy, allowing for earlier decision-making. Ultimately, SVR is the goal of HCV treatment because it dramatically reduces likelihood of poor long-term outcome, even among patients with histologically advanced disease. This article summarizes a basic review presented by Susanna Naggie, MD, at the IAS-USA live management of HCV continuing medical education program held in Atlanta in October 2012. This article is intended for practitioners who are new to HCV management or who are interested in reviewing the basics of HCV treatment.
Overview
This article provides an overview of hepatitis C virus (HCV) disease, including characteristics of the virus, targets for direct-acting antiviral (DAA) drugs, characteristics of virologic response to treatment, background on peginterferon alfa and ribavirin therapy, lessons from the phase II studies of the DAAs telaprevir and boceprevir, and recent pharmacogenetic discoveries.
HCV Infection Characteristics
Epidemiology
Worldwide, approximately 170 million people are infected with HCV, with the highest infection rates found in Asia and Africa. In the United States, the prevalence of infection is approximately 1.6%, which represents 3 to 4 million people, most of whom are unaware of their infection status. An estimated 3.3% of people (4.34% of males, 2.19% of females) in the United States born between 1945 and 1965 have chronic HCV infection (CHC). Recognition of this 3- to 4-fold increased risk compared with the rest of the population has recently led the Centers for Disease Control and Prevention (CDC) to recommend 1-time HCV testing for all persons in this birth cohort. Americans of African descent in this birth cohort are disproportionately affected by HCV, with an infection prevalence of 6.42%, including a prevalence of 13.6% among African American men.1 Approximately 10,000 deaths from HCV-related disease occur annually in the United States, but the numbers of cases of decompensated cirrhosis, hepatocellular carcinoma (HCC), and HCV-related deaths are predicted to dramatically increase with aging of the infected population.
There are 6 genotypes of HCV. Some of these genotypes are associated with poorer response to peginterferon alfa and ribavirin therapy. HCV genotype 1 is the most common in the United States and is associated with the lowest response rate to treatment with peginterferon alfa and ribavirin as well as the longest required treatment duration.
Acute infection often is asymptomatic. Most infected people do not present to health care in the acute phase, with most diagnoses of acute infection occurring when elevated liver enzymes are identified through testing for other reasons. Approximately 75% to 85% of acutely infected people will develop CHC (15%-20% of these will clear the infection spontaneously) and, over the course of a lifetime, 20% of those with CHC will develop cirrhosis. More rapid progression occurs in those who are older at the time of infection, have HIV coinfection, consume more than 50 grams of alcohol daily, or required organ transplantation and immunosuppression. HIV-infected people have a statistically lower chance of spontaneously clearing acute HCV infection and, once they develop chronic infection, have a 70% greater risk of developing cirrhosis than those with HCV monoinfection.2 This discrepancy remains, even in the highly active antiretroviral era.
HCV Genome and Drug Targets
HCV can be eradicated from the body with effective therapy, which may not be possible with other chronic viruses such as HIV or hepatitis B virus (HBV). HCV, a positive-strand RNA virus, lacks a nuclear phase in the viral replication cycle. Unlike HIV, which has an integration phase, or HBV, which replicates via covalently closed circular DNA in the nucleus, HCV lacks a DNA intermediate.
The HCV genome is transcribed and translated into a polyprotein that comprises structural proteins and nonstructural (NS) proteins. The NS proteins are the primary targets of available DAAs. The first US Food and Drug Administration (FDA)-approved DAAs, boceprevir and telaprevir, are NS3/4A protease inhibitors (PIs). “Next-wave” PIs including TMC-435 (simeprevir) and BI-201335 (faldaprevir) are currently in phase III study. Second-generation NS3/4A inhibitors are in phase II testing, with the hope that these new agents will exhibit different resistance profiles that can be exploited in combination strategies and provide options for patients in whom triple-drug combination therapy has recently failed. The NS3/4A protease inhibitors are highly potent but have a low barrier to resistance. Initial monotherapy studies of these agents showed prompt suppression of virus and rapid emergence of resistance, requiring that they be administered along with a peginterferon alfa and ribavirin “backbone” to prevent resistance. It is hoped that combinations of numerous investigational agents with different mechanisms of action will permit all-oral, interferon alfa-free HCV therapies.
The NS5A protein is part of the cytoplasmic replication complex of the virus, but has no known enzymatic activity. Although its function remains unclear, inhibitors of the protein are highly potent inhibitors of viral replication. As with the NS3/4A inhibitors, the high potency is accompanied by a low barrier to resistance. Thus, NS5A inhibitors are likely to find a place in combination therapies, but like NS3/4A inhibitors, they will need to be administered with an agent with a higher barrier to resistance and will require combination regimens to achieve high efficacy.
Inhibitors of the NS5B RNA-dependent RNA polymerase may be able to provide the necessary high barrier to resistance required for a “backbone” drug. Nonnucleoside analogues have exhibited limited potency and a low barrier to resistance, but nucleoside analogues have exhibited moderate to high potency and a very high resistance barrier. One nucleoside analogue currently in phase III study, sofosbuvir (GS-7977), has been studied in more than 1700 chronically infected patients (in combination with peginterferon alfa and ribavirin or ribavirin monotherapy). To date, no on-treatment viral breakthrough has occurred in any subject, but 26 patients have experienced relapse after cessation of sofosbuvir-containing regimens.3 Population sequencing identified only 1 patient (HCV genotype 2b on sofosbuvir monotherapy) with the S282T mutation, which did confer decreased susceptibility to sofosbuvir. Deep sequencing did not identify this mutation in any other patient. These results confirm that sofosbuvir has an exceptionally high barrier to resistance. Data from the phase III program are pending and will further augment the available virology data for sofosbuvir. Future treatment strategies may involve use of an NS5B nucleoside analogue in combination with another high-potency agent(s) with different mechanisms of action.
Ideally, future regimens will have efficacy across all HCV genotypes. The first-generation NS3/4A inhibitors, boceprevir and telaprevir, are primarily active against HCV genotype 1, showing some activity against genotypes 2 and 4, and little to no activity against genotype 3. However, the next-generation investigational NS3/4A inhibitor simeprevir, the novel investigational NS5A inhibitors, and nucleoside analogue NS5B polymerase inhibitors exhibit activity against multiple genotypes and in some cases will be pangenotypic. The nonnucleoside analogues have less broad activity due to noncatalytic binding sites.
On-Treatment Viral Kinetics
Figure 1 shows different types of virologic response as defined for treatment with peginterferon alfa and ribavirin and for use of DAAs.4 Null response to peginterferon alfa and ribavirin is defined as a less than 2-log10 IU/mL reduction in HCV RNA at week 12 of therapy; patients with null response would be discontinued from peginterferon alfa and ribavirin therapy at 12 weeks. Null responders remain more difficult to treat, even with combination DAA regimens. Partial response to peginterferon alfa and ribavirin is defined as achieving at least a 2-log10 IU/mL reduction at week 12, but having persistently detectable HCV RNA at week 24 of therapy. Under prior stopping/futility rules, peginterferon alfa and ribavirin therapy was stopped at 24 weeks in these partial response patients. Relapse indicates an HCV RNA level, which is undetectable at the end of treatment (EOT) but becomes detectable during follow-up. Patients in whom relapse has occurred have excellent response rates with triple-drug therapy, and will likely also respond well to interferon alfa-sparing combinations. Early virologic response (EVR) on peginterferon alfa and ribavirin is defined as at least a 2-log10 IU/mL decline in viral load at week 12 of therapy. This definition is unlikely to be used in the context of treatment containing DAAs, because viral kinetics reveal faster responses with these highly potent regimens.
Figure 1.
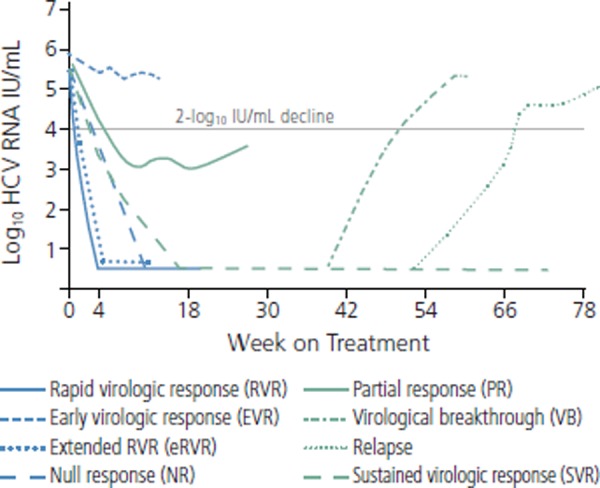
On-treatment viral kinetics and definitions of response to triple-drug treatment (peginterferon alfa ribavirin, and a direct-acting antiviral [DAA]) for hepatitis C virus (HCV). Adapted from Yee et al.4
New definitions of virologic response have arisen with triple-combination therapies, representing predictors of response and a means of determining the length of treatment required (see Box). Rapid virologic response (RVR) indicates an undetectable viral load at week 4 of treatment; this identifies patients with rapid viral clearance kinetics, which is an excellent on-treatment predictor of SVR. Extended RVR (eRVR) indicates an undetectable viral load at week 4 of therapy and maintenance of viral load suppression through week 12; eRVR has been used for response-guided therapy treatment decisions with triple-combination therapies. Very rapid virologic response (vRVR) indicates an undetectable viral load by week 2; this measure may become increasingly important with use of interferon alfa-sparing regimens.
Sustained virologic response (SVR), which is considered to be cure, indicates an undetectable viral load at 12 weeks and 24 weeks after stopping treatment. Breakthrough indicates achievement of undetectable viral load on therapy, followed by an increase to 100 IU/mL or an increase of 1 log10 or greater at any time during treatment. Relapse is defined as detectable HCV RNA during follow-up period in patients with undetectable viral load at the end of treatment.
Because it has been demonstrated that SVR at 12 weeks after EOT (SVR 12) is equivalent to SVR at 24 weeks (SVR 24), SVR 12 has become an acceptable primary endpoint in clinical trials of HCV therapy.
Prior Standard of Care With Peginterferon Alfa and Ribavirin
Representative trials in HCV monoinfection showed SVR rates of 46% with peginterferon alfa-2a and ribavirin, 42% with peginterferon alfa-2b and ribavirin in genotype 1 infection, and 76% and 82%, respectively, in genotype 2 and 3 infection.5,6 The standard duration of peginterferon alfa and ribavirin therapy in patients with genotype 1 infection is 48 weeks, with some patients with delayed viral kinetics receiving 72 weeks of treatment. Peginterferon alfa and ribavirin remains the standard of care in genotype 2 or 3 infection for now, with durations of therapy of 24 weeks or 48 weeks, depending on whether patients are coinfected with HIV and viral kinetics during therapy.
The IDEAL (Individualized Dosing Efficacy vs Flat Dosing to Assess Optimal Pegylated Interferon Therapy) trial, the largest assessing peginterferon alfa and ribavirin therapy in genotype 1 infection, compared 2 doses of peginterferon alfa-2b and peginterferon alfa-2a, each combined with ribavirin. SVR rates were 44% with all 3 treatments among 2189 white patients, 17% to 26% in 570 African American patients, 29% to 44% in 213 Hispanic patients, and 50% to 70% in 51 Asian patients.7 Much of the differences in response rates among racial groups is explained by different prevalences of the IL28B single nucleotide polymorphism associated with improved response to peginterferon alfa and ribavirin therapy (see below).
Several studies of peginterferon and ribavirin therapy were completed in HCV/HIV coinfected patients and showed SVR rates of 14% to 38% in those with genotype 1 or 4 infection and 43% to 73% in those with genotype 2 or 3 infection. In the AIDS Clinical Trials Group (ACTG) 5071 study,8 SVR rates were 14% and 73%, respectively; SVR rates in genotype 1 infection were higher in European studies, likely in part reflecting a higher prevalence of the IL28B polymorphism in European populations.
Overall, baseline clinical characteristics associated with greater likelihood of response to peginterferon alfa and ribavirin in patients with genotype 1 infection have been reported to include lower viral load (eg, < 600,000 IU/mL), less advanced liver fibrosis (stage 0-2), younger age, female sex, non–African American race, lower body weight, less insulin resistance, and less hepatic steatosis. Many of these baseline predictors are likely to lose significance with the availability of DAA-containing regimens that are capable of producing SVR rates of 90% or better. The first rationale for response-guided therapy was derived from findings in the IDEAL trial indicating SVR rates of 84.1%, 74.7%, 70.4%, and 35.5% in patients with undetectable viral load by weeks 2, 4, 12, and 24, respectively.9
Box. Virologic Response Definitions With Triple-Drug Therapy for Hepatitis C Virus Infection
Rapid virologic response (RVR) indicates an undetectable viral load at week 4 of treatment.
Extended RVR (eRVR) indicates an undetectable viral load at week 4 of treatment and maintenance of viral load suppression through week 12; eRVR has been used for response-guided treatment with triple-drug therapy.
Very rapid virologic response (vRVR) indicates an undetectable viral load by week 2; this measure may become important with use of interferon alfa-sparing regimens.
Sustained virologic response (SVR), which is considered to be cure, indicates an undetectable viral load 12 weeks and 24 weeks after stopping treatment. Because it has been demonstrated that SVR at 12 weeks after stopping therapy (SVR 12) is equivalent to SVR at 24 weeks (SVR 24), SVR 12 has become an acceptable primary endpoint in clinical trials of HCV therapy.
Breakthrough indicates achievement of undetectable viral load followed by an increase to 100 IU/mL or an increase of 1 log10 IU/mL or greater at any time during treatment.
Relapse is defined as detectable HCV RNA during follow-up period in patients with undetectable viral load at the end of treatment.
The limitations of peginterferon alfa and ribavirin therapy include poor response rates, long courses of therapy, and adverse events. Common adverse events observed with peginterferon alfa-2a and ribavirin in the IDEAL study are shown in Table 1.9 Triple-drug therapy with the first FDA-approved DAAs has improved response rates and shortened therapy for some patients, but these drugs have expanded adverse event profiles, particularly with regard to anemia and rash.
Table 1.
Common Adverse Events with Peginterferon Alfa and Ribavirin in the IDEAL (Individualized Dosing Efficacy vs Flat Dosing to Assess Optimal Pegylated Interferon Therapy) Trial
| Adverse Event | Proportion Experiencing |
|---|---|
| Fatigue | 63% |
| Headache | 42% |
| Nausea | 36% |
| Insomnia | 41% |
| Pyrexia | 23% |
| Anemia | 34% |
| Myalgia | 22% |
| Neutropenia | 31% |
| Depression | 21% |
| Irritability | 25% |
| Rash | 28% |
Adapted from McHutchison et al.9
The standard therapy in acute HCV infection continues to be peginterferon alfa and ribavirin. Patients have a better chance of cure if they are treated early, with cure being achieved in many patients with acute genotype 1 infection with only 24 weeks of treatment. A recent meta-analysis indicated that SVR is achieved in 82.5% of patients starting treatment within 12 weeks of diagnosis of acute infection, with SVR rates of 66.9% and 62.5% in those starting treatment at 12 weeks to 24 weeks and after 24 weeks, respectively.10 The spontaneous clearance rate in untreated patients was 55.1%, with a mean time to clearance of 9.7 weeks. Thus, studies of acute infection should not start treatment until 12 weeks after diagnosis to permit patients to spontaneously clear infection; otherwise, there is risk of elevating the treatment response rate by including those who would have spontaneously cleared the infection. Improved SVR rates in acute infection have also been observed in patients with HCV/HIV coinfection with reports of 60% to 70%.11,12 Studies are being designed to evaluate DAAs in treatment of acute infection.
Phase II Studies of Boceprevir and Telaprevir: How We Got to Where We Are
Figure 2 shows the designs and outcomes of the phase II SPRINT 1 (Serine Protease Inhibitor Therapy 1) trial of boceprevir and PROVE (Protease Inhibition for Viral Evaluation) 1 and 2 trials of telaprevir in chronically monoinfected treatment naive patients with HCV genotype 1.13-15 A peginterferon alfa and ribavirin lead-in phase was used based on the hypothesis that reduction of viral load with lead-in would reduce risk of emerging resistance when the DAA, in this case boceprevir, was added. The regimen that consisted of a 4-week lead-in followed by 44 weeks of triple-drug therapy produced an SVR rate of 74%, higher than that in the arm receiving 48 weeks of triple-drug therapy (66%). This strategy moved forward into phase III study and now constitutes the current approach to boceprevir-containing therapy. SPRINT 1 also showed that a 48-week course of treatment produced better SVR rates than 28 weeks of treatment, although SVR rates were still greater than 50% with the shorter course of treatment, suggesting that there was a substantial number of patients who could be cured in 28 weeks. Thus, the paradigm of response-guided therapy was born, using early on-treatment viral kinetics to decide which patients could achieve SVR with the shorter course of therapy.
Figure 2.
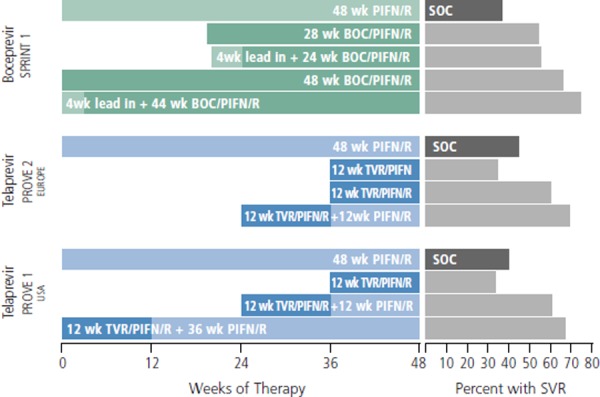
Design and outcomes of phase II trials SPRINT (Serine Protease Inhibitor Therapy) and PROVE (Protease Inhibitor for Viral Evaluation) studies of boceprevir (BOC) and telaprevir (TVR). PIFN indicates peginterferon alfa; R, ribavirin; SOC, standard of care (peginterferon alfa and ribavirin); SVR, sustained virologic response. Compiled from McHutchison et al, Hezode et al, and Kwo et al.13-15
Among the lessons learned from the phase II telaprevir trials is that ribavirin appears to be an essential component of regimens with peginterferon alfa and a single DAA. In the PROVE 2 trial, 12 weeks of treatment with peginterferon alfa plus telaprevir produced an SVR rate of only 36%, lower than the SVR rate with peginterferon alfa and ribavirin (41%). Ribavirin continues to be retained in interferon alfa-sparing regimens, although numerous study designs are assessing the need for ribavirin in combinations of 2 or 3 DAAs. Although the mechanism is not clearly understood, ribavirin appears to reduce rates of resistance emergence and virologic breakthrough. Ribavirin is associated with anemia and other adverse events, but the anemia may be more easily managed in the absence of the marrow suppressive effects of interferon alfa. The PROVE trials also showed that some patients could achieve cure with as few as 12 weeks of triple-drug therapy, with SVR rates of 60% in the European study and 35% in the US study. A post-hoc analysis of the PROVE-2 trial reported that 100% of the patients in the 12-week arm who had the favorable IL28B CC genotype achieved SVR, suggesting that this select patient population may be able to achieve high cure rates with substantially shorter courses of triple-drug therapy.16 With more potent or dual combinations of DAAs in combination with pegIFN/RBV, it may be possible to achieve SVR in such patients with as few as 6 weeks of treatment.17
The current criteria for viral monitoring of triple-drug therapy with a DAA incorporate response-guided therapy, which can shorten therapy in 45% to 60% of patients, and new stopping rules that spare patients exposure to futile therapy and excess cost (Figure 3).18 For telaprevir, treatment-naive patients or those who have relapsed after prior therapy receive triple-drug therapy for 12 weeks, followed by an additional 12 weeks or 36 weeks of peginterferon alfa and ribavirin, depending on the early virologic response. Patients with eRVR (undetectable HCV RNA at 4 weeks and 12 weeks) receive 12 additional weeks of peginterferon alfa and ribavirin. Those with detectable virus of 1000 IU/mL or less at week 4 and/or week 12 receive 36 additional weeks of peginterferon alfa and ribavirin. Patients with prior partial and null responses and patients with cirrhosis should receive 12 weeks of triple-drug therapy and 36 additional weeks of peginterferon alfa and ribavirin; response-guided therapy is not recommended in these more difficult-to-treat populations. Stopping therapy is recommended in all patients with HCV RNA greater than 1000 IU/mL at week 4 or 12 or detectable HCV RNA at week 24.19
Figure 3.
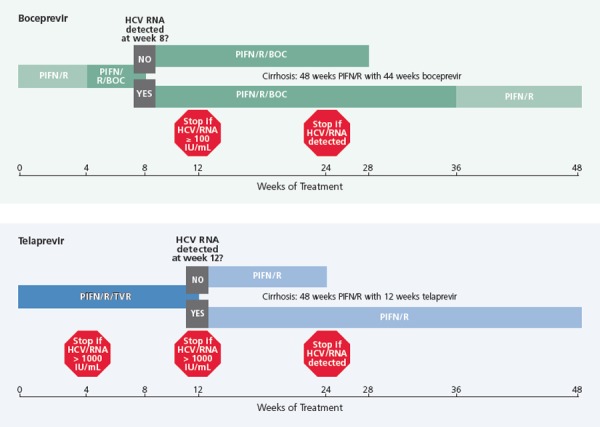
Stopping (futility) rules for triple-drug therapy including boceprevir (BOC; top) or telaprevir (TVR; bottom) in treatment-naive patients. HCV indicates hepatitis C virus; PIFN/R, peginterferon and ribavirin. Adapted from Barritt et al.18
It is important to note that if virologic breakthrough occurs on telaprevir-containing triple-drug therapy, it is likely to occur before week 12 of treatment. Patients with a HCV RNA level at week 4 of 600 IU/mL, for example, can already be identified as slow responders at greater risk of resistance emergence. It makes sense to monitor HCV RNA more frequently; for example, every 2 weeks—in such patients, in order to avoid delayed recognition of treatment failure, which could allow increased emergence of resistant mutants. Although it is unclear what resistance to PIs portends for treatment in the long run, it is known that resistance mutations can persist for up to 18 months.20 Thus, patients with a resistant virus may be precluded from receiving HCV NS3/4A-containing combinations for 18 months to 2 years.
For boceprevir, patients first receive a 4-week lead-in with peginterferon alfa and ribavirin and then begin triple-drug therapy. For treatment-naive patients and for patients with previous partial responses or relapse, those with undetectable HCV RNA at both week 8 and week 24 can stop therapy early at week 28 and week 36, respectively. Those with detectable HCV RNA at week 8 but undetectable HCV RNA at week 24 receive triple-drug therapy through week 36 and then continue only the peginterferon alfa and ribavirin through week 48. For all patients, the stopping rules are to cease triple-drug therapy if HCV RNA is 100 IU/mL or greater at week 12 or HCV RNA is detectable at week 24. Response-guided therapy was not studied in prior null responders; if such patients are treated, they should receive 4 weeks of peginterferon alfa and ribavirin followed by 44 weeks of triple-drug therapy; this is the same treatment recommendation to use for all patients with cirrhosis.21
As with slow responses to telaprevir-containing triple-drug therapy, it makes sense to monitor viral load every 2 weeks in patients receiving boceprevir who have detectable HCV RNA below 100 IU/mL at week 12, until they achieve full viral suppression.
Pharmacogenetic Discoveries
IL28B Genotypes
A genome-wide association study conducted using the IDEAL trial population to investigate more than 600,000 single nucleotide polymorphisms (SNPs) reported that 1 SNP (rs12979860) localizing just upstream of the IL28B gene was strongly associated with treatment response to peginterferon alfa and ribavirin therapy in treatment-naive patients with genotype 1 infection.22 The findings were subsequently confirmed in Japanese and Australian cohorts23,24 and then confirmed to also be associated with spontaneous clearance of HCV infection.25
As shown in Figure 4, the favorable IL28B CC genotype was associated with significantly greater likelihood of SVR than were the less favorable CT/TT genotypes in whites of European descent, African Americans, and Latinos.22 The coefficient of determination for this association was 0.5, indicating that 50% of the variability in response to treatment was explained by this single SNP. A subsequent analysis in a different cohort of patients showed that the CC genotype was also associated with an approximately 3-fold higher rate of spontaneous clearance of HCV than other genotypes, with clearance occurring in more than 50% of patients of European descent and patients of African descent.25 The CC genotype was also associated with a 3.7-fold increased likelihood of SVR in HCV/HIV coinfected patients.26 The IL28B C allele occurs with a frequency of 90% to 100% in East Asia, 25% to 40% in Africa, 50% to 75% in Europe, 60% to 80% in South America, and 30% to 60% in North America.25
Figure 4.
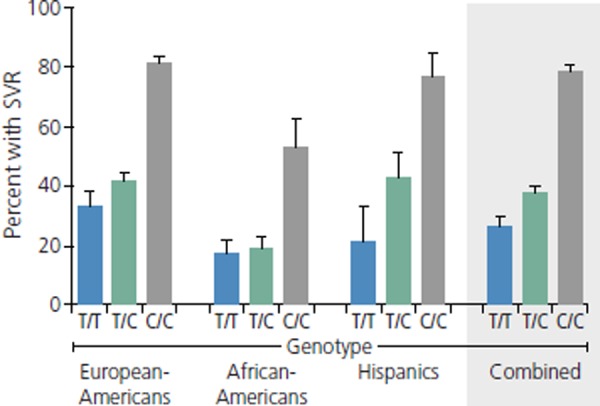
Proportions of patients with sustained virologic response (SVR) according to IL28B genotype and race/ethnicity. Adapted from Ge et al.22
The IL28B gene encodes for interferon lambda. Like interferon alfa, it is an endogenous cytokine with antiviral activity, including activity against HCV. Both cytokines have receptors on epithelial cells, including cells in the liver. However, unlike interferon alfa, interferon lambda does not have receptors in bone marrow cell lines, and is not associated with the cytopenias caused by interferon alfa treatment. Interferon lambda is currently in phase III studies for treatment of HCV infection.
Inosine Triphosphate Pyrophosphatase (ITPA)
Analysis in the IDEAL trial population also showed that 2 SNPs in the ITPA gene (rs1127354 CC and rs7270101 AA) were highly associated with increased risk of anemia in patients receiving peginterferon alfa and ribavirin.27 Similar findings were made in a cohort of HCV/HIV coinfected patients.28 It has not been found, however, that the increased risk of anemia is associated with reduced likelihood of SVR in either HCV-monoinfected or HCV/HIV coinfected patients, thus the clinical utility of this finding remains questionable.
What Does SVR Mean for Patients’ Long-Term Outcomes?
SVR is a virologic endpoint of therapy. Ability to achieve SVR translates into greatly reduced risk of liver disease–related clinical outcomes, including end-stage liver disease, decompensation, HCC and need for liver transplantation. In a cohort of 638 HCV/HIV coinfected patients (80% black, 66% men), no end-stage liver disease, HCC, or death was observed for up to 10 years of follow-up in patients achieving SVR (n=36) or in those with relapse (n=15) (Figure 5).29 The findings in patients who had relapse suggest that having undetectable virus for a year with peginterferon alfa and ribavirin therapy, even with relapse, portends good long-term outcome. Risk for these events did not differ between patients with nonresponse to therapy and those who did not receive treatment.
Figure 5.
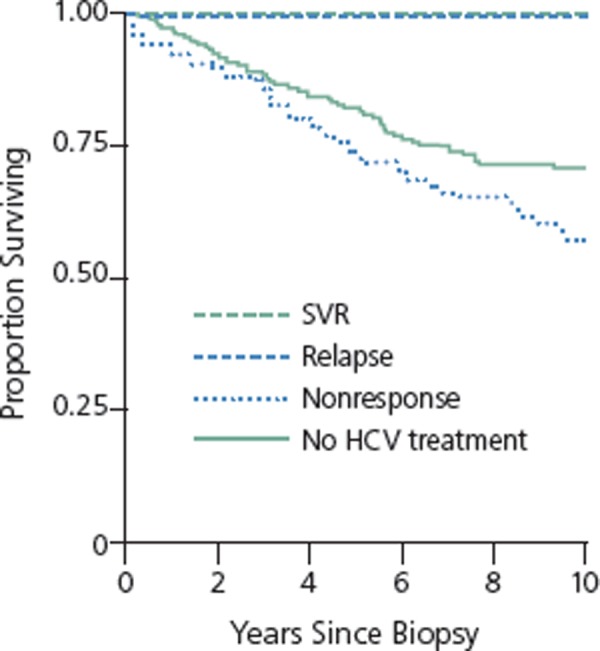
Survival according to sustained virologic response (SVR), relapse, nonresponse, or no treatment in hepatitis C virus (HCV)/HIV coinfected patients at Johns Hopkins HIV Clinic. Adapted from Limketkai et al.29
Follow-up of patients with histologically advanced CHC in the HALT-C (Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis) trial showed that patients with SVR had statistically significantly reduced risk for death from any cause or liver transplantation, any liver-related outcome, decompensated liver disease, HCC, and liver-related death or transplantation compared with nonresponders followed for up to 7.5 years (Figure 5).30
Summary
The past 2 years have been very exciting in the field of HCV management and treatment. We have seen the approval of the first DAAs for HCV and the proof that HCV can be cured without the use of interferon alfa. As numerous DAAs move into phase III study, including at least 3 programs with all-oral interferon alfa-sparing combinations, great hope exists for our patients with CHC. As we move forward, we hope to gain more information on the safety and efficacy of these highly potent combination therapies in more difficult-to-treat patients such as those with HIV coinfection, cirrhosis, severe liver disease, renal failure, and liver transplantation. The next 5 years are sure to bring many exciting discoveries as we work to eradicate HCV and prevent or slow the progression of liver disease in the millions of people around the globe with this deadly chronic infection.
References
- 1.Armstrong GL, Wasley A, Simard EP, Mc-Quillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705-714. [DOI] [PubMed] [Google Scholar]
- 2.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22(15):1979-1991. [DOI] [PubMed] [Google Scholar]
- 3.Svarovskaia ES, Dvory-Sobol H, Gontcharova V, Chiu S, Hebner C, et al. Comprehensive resistance testing in patients who relapsed after treatment with sofosbuvir (GS-7977)-containing regimens in phase 2 studies. [Abstract 753.] 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD) November 9-13, 2012; Boston, MA. [Google Scholar]
- 4.Yee HS, Chang MF, Pocha C, et al. Update on the management and treatment of hepatitis C virus infection: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program Office. Am J Gastroenterol. 2012;107(5):669-689. [DOI] [PubMed] [Google Scholar]
- 5.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982. [DOI] [PubMed] [Google Scholar]
- 6.Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965. [DOI] [PubMed] [Google Scholar]
- 7.Muir AJ, Hu KQ, Gordon SC, et al. Hepatitis C treatment among racial and ethnic groups in the IDEAL trial. J Viral Hepat. 2011;18(4):e134-e143. [DOI] [PubMed] [Google Scholar]
- 8.Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351):451-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361(6):580-593. [DOI] [PubMed] [Google Scholar]
- 10.Corey KE, Mendez-Navarro J, Gorospe EC, Zheng H, Chung RT. Early treatment improves outcomes in acute hepatitis C virus infection: a meta-analysis. J Viral Hepat. 2010;17(3):201-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews GV, Hellard M, Haber P, et al. Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis. 2009;48(5):650-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogel M, Nattermann J, Baumgarten A, et al. Pegylated interferon-alpha for the treatment of sexually transmitted acute hepatitis C in HIV-infected individuals. Antivir Ther. 2006;11(8):1097-1101. [PubMed] [Google Scholar]
- 13.McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360(18):18271838. [DOI] [PubMed] [Google Scholar]
- 14. Hézode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360(18):1839–1850.. [DOI] [PubMed] [Google Scholar]
- 15.Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376(9742):705-716. [DOI] [PubMed] [Google Scholar]
- 16.Bronowicki JP, Hezode C, Bengtsson L, et al. 100% SVR in IL28B CC patients treated with 12 weeks of telaprevir, peginterferon and ribavirin in the PROVE2 trial. J Hepatol. 2012; 56(Suppl 2):S430-S431. [Google Scholar]
- 17.Thompson AJ, Shiffman ML, Rossaro L, et al. Six weeks of an NS5A Inhibitor (GS-5885) and a protease inhibitor (GS-9451) plus peginterferon + ribavirin achieves high SVR4 rates in genotype 1 IL28B CC treatment-naive hepatitis C virus patients: interim results of a prospective, randomized trial. [Abstract 759.] 63rd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD) November 9-13, 2012; Boston, MA. [Google Scholar]
- 18.Barritt AS, Fried MW. Maximizing opportunities and avoiding mistakes in triple therapy for hepatitis C virus. Gastroenterology. 2012;142(6):1314-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Telaprevir [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. [Google Scholar]
- 20.Susser S, Vermehren J, Forestier N, et al. Analysis of long-term persistence of resistance mutations within the hepatitis C virus NS3 protease after treatment with telaprevir or boceprevir. J Clin Virol. 2011;52(4):321-327. [DOI] [PubMed] [Google Scholar]
- 21. Boceprevir [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; 2011. [Google Scholar]
- 22.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399-401. [DOI] [PubMed] [Google Scholar]
- 23.Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41(10):1100-1104. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41(10):1105-1109. [DOI] [PubMed] [Google Scholar]
- 25.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265):798-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rallon NI, Naggie S, Benito JM, et al. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in HIV/hepatitis C virus-coinfected patients. AIDS. 2010;24(8):F23-F29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fellay J, Thompson AJ, Ge D, et al. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature. 2010;464(7287):405-408. [DOI] [PubMed] [Google Scholar]
- 28.Naggie S, Rallon NI, Benito JM, et al. Variants in the ITPA gene protect against ribavirin-induced hemolytic anemia in HIV/HCV-coinfected patients with all HCV genotypes. J Infect Dis. 2012;205(3):376–383.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limketkai BN, Mehta SH, Sutcliffe CG, et al. Relationship of liver disease stage and antiviral therapy with liver-related events and death in adults coinfected with HIV/HCV. JAMA. 2012;308(4):370-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan TR, Ghany MG, Kim HY, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52(3):833-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional Suggested Resources
Review Articles
- •.Sherman KE. Advanced liver disease: what every hepatitis c virus treater should know. Top Antivir Med. 2011;19(3):121125. [PMC free article] [PubMed] [Google Scholar]
- •.Rice CM. New insights into HCV replication: potential antiviral targets. Top Antivir Med 2011;19(3):117-120. [PMC free article] [PubMed] [Google Scholar]
- •.Thomas DL. Advances in the treatment of hepatitis c virus infection. Top Antivir Med. 2012;20(1):5-10. [PMC free article] [PubMed] [Google Scholar]
- •.Schooley RT. Hepatitis c virus therapeutics: at the end of the beginning. Top Antivir Med. 2012;20(1):17-19. [PMC free article] [PubMed] [Google Scholar]
- •.Sherman KE. Managing adverse effects and complications in completing treatment for hepatitis c virus infection. Top Antivir Med. 2012;20(4):125-128. [PMC free article] [PubMed] [Google Scholar]
- •.Wyles DL. Beyond telaprevir and boceprevir: reistance and new agents for hepatitis c virus infection. Top Antivir Med. 2012;20(4): 139-145. [PMC free article] [PubMed] [Google Scholar]
Website
- •.University of Liverpool HIV Pharmacology Group. HIV Drug Interactions. http://www.hiv-druginteractions.org/. Accessed December 20, 2012. [Google Scholar]
Continuing Medical Education Activities
- •.Issues in the care of HIV and hepatitis C virus-coinfected patients: antiretroviral pharmacokinetics, drug interactions, and liver transplantation. Cases on the Web. International Antiviral Society-USA. http://cows.iasusa.org/cow-instructions.php?cowid=102. Accessed November 29, 2012. [Google Scholar]
- •.New treatments for hepatitis c virus infection. Cases on the Web. International Antiviral Society-USA. http://cows.iasusa.org/cow-instructions.php?cowid=184. Accessed November 29, 2012. [Google Scholar]
- •.Drug interactions with medications for treating hepatitis c virus infection. Cases on the Web. International Antiviral Society-USA. http://cows.iasusa.org/cow-instructions.php?cowid=231. Accessed November 29, 2012. [Google Scholar]
- •.Management of chronic hepatitis c virus infection in advanced liver disease. Cases on the Web. International Antiviral Society-USA.http://cows.iasusa.org/cow-instructions.php?cowid=264. Accessed November 29, 2012. [Google Scholar]
- •.The use of hepatitis c virus (HCV) protease inhibitors in HIV/HCV-coinfected patients. Cases on the Web. International Antiviral Society-USA.http://cows.iasusa.org/cow-instructions.php?cowid=279. Accessed November 29, 2012. [Google Scholar]


