Abstract
Splenectomized mice express progressively increased numbers of platelets in the blood and reduced numbers of megakaryocytes in the marrow with age. The megakaryocytes in the marrow of these animals express reduced levels of Gata1, a transcription factor necessary for their maturation. In addition, the marrow from these animals expresses greater levels of cytokines (TGF-β, PDGF-α, and VEGF) known to be produced at high levels by megakaryocytes expressing reduced levels of Gata1. This high level of cytokine expression is in turn associated with active osteoblast proliferation localized to areas of the femur, where megakaryocytes expressing reduced Gata1 levels are also found. These results confirm the role of megakaryocytes as regulator of bone formation in mice and suggest that a cross-talk between the spleen and marrow may regulate the total numbers of hemopoietic niches present in an animal.
Keywords: spleen, megakaryocytes, Gata1, bone formation
Introduction
The ability of the spleen to support clonal maturation of hematopoietic stem/progenitor cells provided the basis for the first quantitative stem cell assay, the spleen focus forming colony assay.1 In mice, however, the spleen is dispensable for hematopoiesis under steady-state conditions and is recruited as a hematopoietic site mainly under conditions of stress.2–6 Under normal circumstances, hematopoiesis is restricted to the marrow, where it is carefully regulated by the balanced interaction between hematopoietic stem/progenitor cells and their niches.7–9
In recent years, great progress has been made in the definition of the hematopoietic niches in the marrow.7–9 At least two distinct hematopoietic marrow niches have been recognized: the osteoblast niche, which hosts the hematopoietic stem cells, and the vascular niche, which supports the process of stem cell commitment into lineage-restricted progenitor cells.7–9 Much less is known about the nature of the niches present in extramedullary sites such as the spleen. Given the high vascularization of the spleen, it is conceivable that this organ contains vascular niches.10,11 It is debatable, however, whether the biological properties of the vascular niche in the spleen are similar to those expressed by the corresponding niche in the marrow. In fact, while the marrow vascular niche supports cell maturation toward multiple hematopoietic lineages, the spleen niche preferentially supports erythroid maturation.2,3,10 Although the spleen does not have osteoblasts, it contains mesenchymal stem cells capable of differentiating into this lineage in vitro12 and in vivo, by forced expression of IL-5.13 The reason osteoblasts do not normally differentiate in vivo in the spleen has been ascribed to the high numbers of lymphocytes present in this organ that may exert an inhibitory effect on osteoblast differentiation.14
Recently, it has been suggested that the total numbers of stem cell niches may limit the total number of stem cells generated in an animal. Experimental manipulations that increase (ectopic increases of bone mass by grafting osteoblastic cell lines15) or decrease (treatment with granulocyte colony-stimulating factor [G-CSF]16,17 and conditional ablation of osteoblast activity18) the number of osteoblasts increase and decrease, respectively, the number of stem cells present in the animals. Whether the niches present in the spleen contribute to determining the total stem cell numbers in an animal is not known.
Splenectomy is commonly considered a mild procedure that does not induce major consequences in mice. To clarify whether the spleen affects the marrow micro-environment, we analyzed the long-term effects of removal of the spleen on hematopoiesis in mice. Wild-type CD1 mice were splenectomized at 6 months of age, and the morphological features and cytokine profile expressed by the marrow were analyzed for 9 months after surgery. In contrast to common knowledge, the results indicated that removal of the spleen has considerable long-term consequences in mice. It selectively increases the number of platelets present in the circulation, and it reduces the number of megakaryocytes present in the marrow and the levels of Gata1 expressed by these cells. In addition, splenectomy increases the levels of a number of cytokines usually expressed by immature megakaryocytes19 and stimulates osteoblast proliferation. These results suggest the existence of cross-talk between the spleen and marrow micro-environment that balances the number of stem cell niches present in the two organs.
Materials and Methods
Mice
CD1 females were purchased from Charles River (Calco, Italy) and housed for up to 2 yr under good animal care practice conditions in the animal facilities of Istituto Superiore Sanità. All experiments were performed with sex- and age-matched mice under protocols approved by the institutional animal care committee.
Splenectomy
Eleven CD1 female mice were anesthetized with xylazine (10 mg/kg, Bayer, Milan, Italy) and ketamine (200 mg/kg, Gellini Farmaceutics, Latina, Italy) i.p. 1 day following food withdrawal. The spleen was removed after double ligation of the splenic artery and vein. The muscle, peritoneum, and skin were closed in separate layers using sterile 5–0 absorbable suture. Animals received the analgesic butorphanol s.c. (5 mg/kg/day, Intervet Italia Srl, Milan, Italy) for 4 days postsurgery. All the mice remained alive for the first week after the surgery; 3 mice died in the first month, but 6 mice were still alive 9–10 months after surgery.
Hematological Parameters
Blood was collected from the retro-orbital plexus into ethylen-diamino-tetracetic acid-coated microcapillary tubes (20–40 μL/sampling). Hematocrit (Hct), white cells (WBC) and platelets (ptl) counts were determined manually.
Histology
Femurs were fixed in 10% (v/v) phosphate-buffered formalin (Sigma, St. Louis, MO, USA), paraffin embedded, and cut into 2.5–3 μmol/L sections that were stained with hematoxylin-eosin or Mallory-trichromic staining (Bio-optica, Milano spa, Italy).20 Microscopic evaluations were performed with a DM RB microscope (Leica LTD, Heidelberg, Germany) set in a transillumination mode, and images were acquired with the IM 50 system (Leica). The number of megakaryocytes was determined at 40x original magnification in randomly chosen multiple sections to cover a total area of 33.5 mm2. For immunohistochemistry, sections of 4–6 μm were incubated with an anti-Gata1 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), and binding was revealed by the avidin-biotin immunoperoxidase system (Vectastain Elite ABC Kit; Vector Laboratories, Burlingame, CA, USA), as described by the manufacturers. Samples not incubated with the primary antibody or incubated with a nonimmune IgG served as negative controls. Samples were counterstained with hematoxylineosin and analyzed with a light microscope (Leica) equipped with a Coolsnap videocamera for computerized images (RS Photometrics, Tucson, AZ, USA).
RNA Isolation and Semiquantitative and Quantitative RT-PCR Analysis
Total RNA was prepared from bone marrow cells lysed in Trizol (Gibco BRL, Paisley, UK). RNA (1 μg) was reverse transcribed with 2.5 μmol/L random hexamers using the superscript kit (InVitrogen, Milan, Italy). Osteocalcin, Acethyl-colinesterase E, transforming growth factor-β (TGF-β1), platelet-derived growth factor-α (PDGF-α), and vascular endothelial growth factor (VEGF) cDNA were quantified by semiquantitative RT-PCR, as described.19 Glyceraldehyde 3-phosphase dehydrogenase (GAPDH) cDNA was concurrently amplified as a housekeeping control. Reactions were performed in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) using the following parameters: 40 cycles of a two-step PCR program at 95°C for 15 sec and 60°C for 60 sec, after an initial denaturation/activation step at 95°C for 10 min. For semiquantitative RT-PCR determinations, aliquots were harvested from the amplification reactions after 20, 25, 30, and 35 cycles and separated on agarose gel.
Statistical Analysis
Statistical analysis was performed by analysis of variance (ANOVA) using Origin 3.5 software for Windows (Microcal Software Inc., Northampton, MA).
Results
Removal of the Spleen Increases the Number of Platelets in the Blood and Reduces the Number of Megakaryocytes in the Marrow
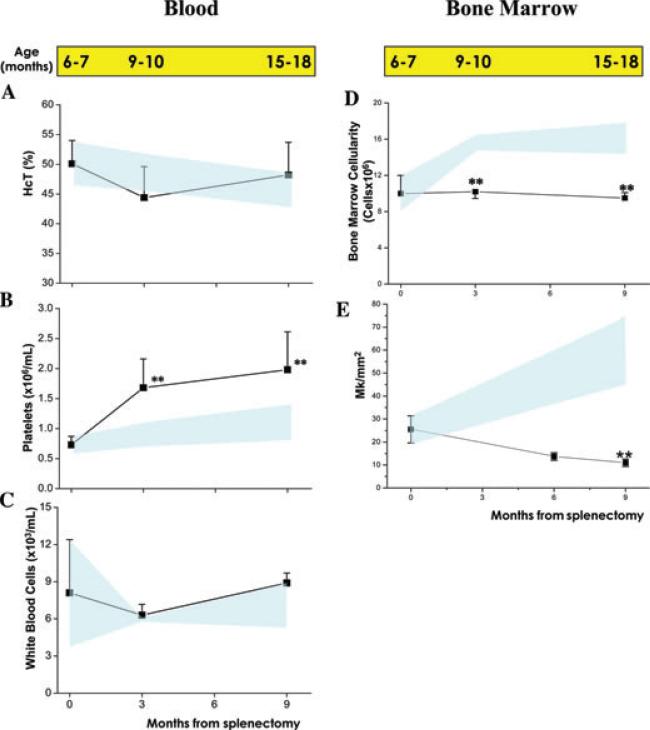
In the blood of untreated animals, a slight decrease in hematocrit and increase in platelet numbers (both not statistically significant) was observed with age, while the numbers of white blood cells remained constant (Fig. 1A–C). On the other hand, in the blood of splenectomized animals, the platelet counts significantly increased by 2–3-fold with age, while no changes in Hct and WBC counts were detected.
Figure 1.
Splenectomy significantly increases platelet counts in the blood and reduces the number of megakaryocytes in the bone marrow of wild-type mice. Hematocrit (Hct), platelet, and white blood cell counts in wild-type mice at different time points after splenectomy are presented in A, B, and C, respectively. Bone-marrow cellularity and frequency of megakaryocytes in the same animals are presented in D and E. The corresponding age of the mice is reported on the top, for comparison. Results are expressed as mean (± SD) of at least 5–7 animals per experimental group and are compared with those expressed by untreated wild-type mice of comparable age (light blue area). Values statistically different (P < 0.01) from the corresponding untreated controls are indicated by **.
In untreated animals, aging was also associated with progressive increase of marrow cellularity (from approximately 9 × 106 cells/femur to 16–17 × 106 cells/femur, Fig. 1D). By contrast, the number of nucleated cells in the marrow of splenectomized mice did not increase with age and remained significantly lower than that of the control nonsplenectomized group of comparable age (Fig. 1D). Although the frequency of megakaryocytes in untreated wild-type mice progressively increased with age, the frequency of megakaryocytes in the marrow of the splenectomized mice progressively decreased with age from an average of 25 megakaryocytes/mm2 observed at 6–7 months to less than 10 megakaryocytes/mm2 at 15–18 months (P < 0.01, Fig. 1E).
In conclusion, removal of the spleen affected megakaryocytopoiesis in mice by increasing the number of platelets in the blood and reducing the number of megakaryocytes present in the marrow. These results suggest that splenectomy accelerates megakaryocyte maturation in the marrow.
Removal of the Spleen Is Associated with Decreased Gata1 Expression in Megakaryocytes
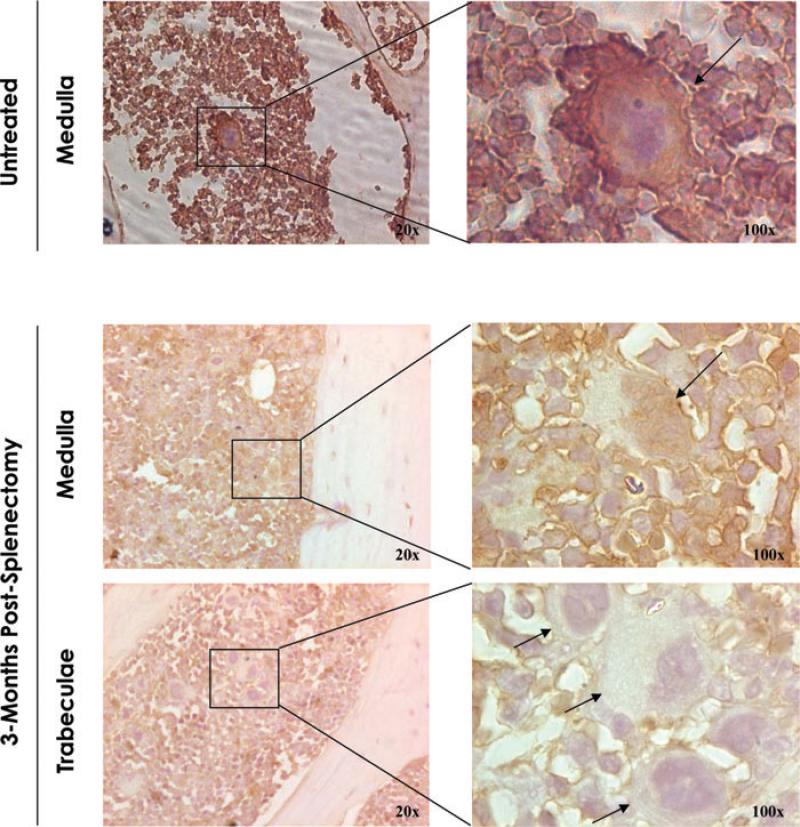
The process of megakaryocyte maturation is exquisitely guided by the transcriptional factor Gata1.21 To clarify the reason for the increased numbers of platelets in the blood after splenectomy, we compared by immunochemistry the Gata1 content of the megakaryocytes present in the marrow of untreated and splenectomized mice. In untreated mice, megakaryocytes were predominantly located within the medulla, and the nuclear area of these cells reacted strongly, as expected, with the anti-Gata1 antibody (Fig. 2). By contrast, a significant fraction (~50%) of the megakaryocytes from splenectomized mice did not react with the anti-Gata1 antibody. In addition, these Gata1neg megakaryocytes were preferentially located in proximity to the endosteum and bone trabeculae. The low Gata1 content of the megakaryocytes present in the marrow after splenectomy suggests that these cells may be immature.
Figure 2.
Megakaryocytes from splenectomized mice express reduced Gata1 content and are localized in areas close to bone trabeculae. Gata1 immunostaining of sections of the long bones from untreated (top panels) and splenectomized (bottom panels) wild-type mice, as indicated. In the case of splenectomized mice, representative areas of the medulla and close to the bone trabeculae are represented independently. In untreated mice, megakaryocytes were detected in the medullary portion of the marrow and were mostly positive for Gata1 (Gata1pos) by immunohistochemistry. By contrast, 3 months after splenectomy, numerous Gata1neg megakaryocytes were detected in marrow sections organized in clusters of cells localized within bone trabeculae. Results are representative of those observed with three additional mice per experimental point. Rectangles indicate areas presented in the enlargements on the right.
Removal of the Spleen Alters the Cytokine Expression Profile of the Marrow
To confirm that reduced expression of Gata1 in megakaryocytes after splenectomy delayed their maturation, we compared the levels of acethylcholinesterase E, a gene specifically expressed by megakaryocytes at early stages of maturation,22 expressed in the marrow of untreated and splenectomized mice. The marrow of mice with impaired megakaryocyte maturation due to reduced Gata1 expression is characterized by high expression levels of several cytokines, including TGF-β, PDGF-α, and VEGF.19 Therefore, to analyze whether the delayed maturation of megakaryocytes had altered the cytokine expression profile in the marrow, we compared the levels of TGF-β, PDGF-α, and VEGF expressed by the marrow of untreated and splenectomized animals. In addition, since the level of expression of osteocalcin is a biomarker for bone formation,23–25 the levels of osteocalcin expressed in the micro-environment of these animals were measured as well.
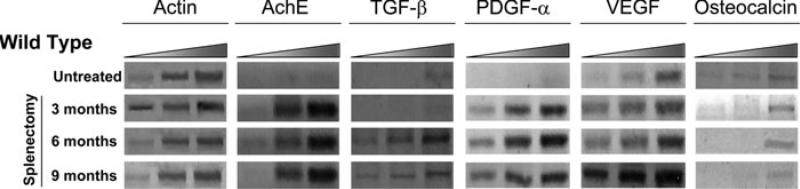
As predicted, in spite of the fact that the marrow of splenectomized mice contains lower numbers of megakaryocytes, the levels of acethylcholinesterase E expressed in this tissue is drastically increased, an indication that these megakaryocytes are indeed less mature (Fig. 3). The marrow from splenectomized wild-type animals expresses much higher levels of TGF-β, PDGF-α, and VEGF than those expressed by untreated mice (Fig. 3). The expression of PDGF-α and VEGF increased as early as 3 months after splenectomy, while the increases in TGF-β expression were observed 6 months after surgery. By contrast, the expression of osteocalcin was not affected by the removal of the spleen (Fig. 3).
Figure 3.
The marrow from splenectomized wild-type mice expresses increased levels of acetylcholinesterase E, TGF-β, PDGF-α, and VEGF. Semiquantitative RT-PCR analysis for the levels of acetylcholin esterase E (AchE), TGF-β, PDGF-α, VEGF, and osteocalcin expressed by the marrow from untreated wild-type mice and in their littermates splenectomized 3, 6, and 9 months earlier, as indicated. Each product was amplified for increasing number of cycles (20, 25, 30, and 35), as indicated by the triangle on top. Actin was amplified as control. Similar results were obtained with two additional mice per experimental point.
Removal of the Spleen Is Associated with Increased Bone Formation
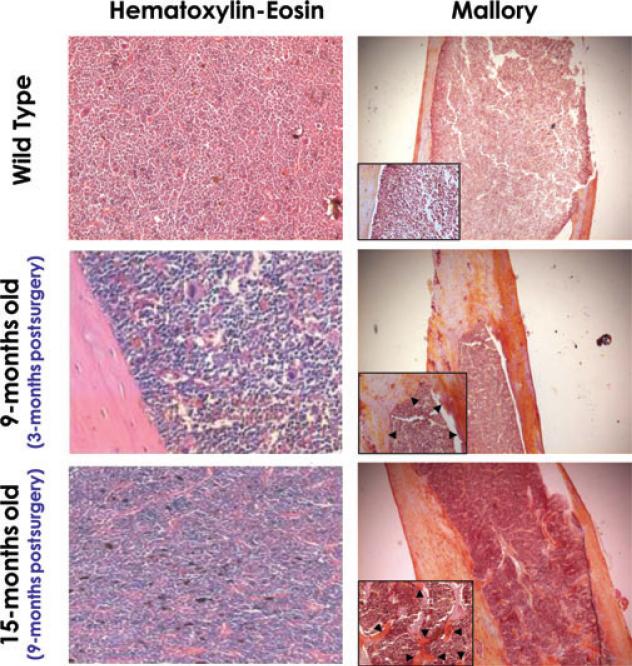
In mice by 6 months of age, osteogenesis is completed and very few areas of the bones react with Mallory staining, an indication that areas of active bone proliferation are almost absent in these mice. Bone formation is the result of a balance between the positive and the negative action exerted by osteoblasts and osteoclasts, respectively. In untreated wild-type mice, the thickness of the bones progressively decreases while their reactivity with Mallory staining remains poor with age (results not shown). By contrast, after splenectomy, the thickness of the bones progressively increases with age and numerous bone trabeculae protrude into the bone cavity 9 months postsurgery. In addition, both the bone areas just above the endoesteum and the trabeculae react strongly in yellow with Mallory staining, an indication of active osteo-genesis (Fig. 4).
Figure 4.
Splenectomy induces bone formation increasing the thickness of the bone walls and the number of trabeculae present within the medulla. Hematoxylin-eosin (left panels) and Mallory (right panels) staining of long bones from representative 12- to 15-month-old wild-type mice (top panels) and from wild-type mice 3 (middle panels) and 9 months (bottom panels) after splenectomy. The age of the mice is indicated, for convenience. Splenectomy did not change the marrow cellularity, but increased the thickness of the bones, the numbers of trabeculae infiltrating the bone cavity, and the staining (in yellow) after Mallory (arrowheads). Results are representative of those obtained in two untreated wild-type and in three (3-month postsurgery) and two (9-month postsurgery) splenectomized mice, respectively. Magnification is 20× in the panels and 40× in the inserts.
Discussion
It is common knowledge that the spleen plays a negligible role in hematopoiesis under steady-state conditions. By contrast, we observe that the number of platelets in the circulation of mice following splenectomy is significantly greater than that present in the blood from untreated controls (Fig. 1). This increase is probably due to accelerated maturation of megakaryocytes because the frequency of these cells in the marrow is reduced. This hypothesis is further supported by the reduced levels of Gata1, a transcription factor necessary for megakaryocyte maturation21 and increased levels of acethylcoholinesterase E, a marker of early megakaryocyte maturation stages22 expressed in the marrow of splenectomized mice (Figs. 2 and 3). These results suggest that under steady-state conditions the spleen releases either soluble factors or cells that specifically reduce the speed of megakaryocyte maturation in the marrow.
The block in megakaryocyte maturation induced by experimental mutations reducing the expression of either Gata1 (Gata1low mutation) or p45-NF-E2, another transcription factor important for the maturation of this lineage (NF-E2null mutation) results in increased release in the marrow micro-environment of several cytokines (such as TGF-β, VEGF, and PDGF-α).19,26,27 Some of these factors induce osteoblast proliferation, and the bones of these mouse mutants are significantly thicker than those of normal mice.26,27 In fact, the differentiation process of mesenchymal stem cells into mature osteoblasts occurs in three sequential phases: proliferation, matrix maturation (detected by Mallory), and mineralization.23,24
At the onset of the mineralization phase, osteoblasts upregulate the expression of osteocalcin, a noncollagenous protein that regulates calcium homeostasis.25 Gata1low and NF-E2null megakaryocytes are able to support osteoblast maturation until the matrix maturation phase, as indicated by the strong reaction of the bones from these mutants with Mallory, a staining specific for the proteins of the bone matrix,20 but fail to sustain the mineralization phase, as indicated by the fact that the femurs of the mutant mice express low levels of osteocalcin19 and are frail and prone to fracture upon manipulation.26,27 Since accumulation of immature megakaryocytes in the marrow of mice has been observed up to now only as consequence of genetic manipulations, it is not known whether it may also be induced by physiologic stimuli. We describe here that the accumulation of immature megakaryocytes in the marrow induced by removing the spleen is indeed associated with increased levels of TGF-β, VEGF, and PDGF-α expression in the marrow micro-environment similar to those induced by the Gata1low and the 45-NF-E2null mutation (Fig. 3 and Ref. 19). In addition, removal of the spleen increases the thickness of the bones, probably due to increased osteoblast proliferation (Fig. 4). Also in this case, osteoblast maturation progressed up to the mineralization phase, as the expression of osteocalcin remained low (Fig. 3) and the bones became fragile after manipulation (results not shown). These results suggest that the same soluble or cellular factors produced by the spleen that regulate megakaryocyte maturation inhibit the proliferation of the osteoblasts, limiting the number of stem cell niches available in the bones.
Several hematopoietic cells are known to regulate the number of osteoblasts, and therefore of stem cell niches, present in the marrow.28 A population of resident macrophages has been described that line the endosteum and regulate osteoblast proliferation.29 In addition, macrophages by differentiating into osteoclasts exert a negative control on the number of osteoblasts present in the bone.30 The observations described in this manuscript confirm that megakaryocytes at early stage of maturation secrete growth factors that positively control osteoblast proliferation and therefore control the number of stem cell niches present in the marrow.
Splenectomy is a common palliative therapy for numerous pathological conditions such as the symptomatic splenomegaly refractory to other treatments associated with the myeloproliferative disorder primary myelofibrosis (PMF).31 However, splenectomy in patients with PMF has been associated with a higher incidence of development of extramedullary hematopoiesis in liver and higher rate of blastic transformation.31 It is, therefore, debatable whether the involvement of the spleen in the pathogenesis of PMF is secondary to hematopoietic failure in the marrow or whether the spleen, by providing a specific micro-environment, plays an active role in restraining the progression of the disease.32 The long-term alterations described here to occur in the marrow of normal mice after splenectomy suggest that the full spectrum of the consequences of removal of spleen in PMF patients, as well as in other human diseases, deserves to be carefully evaluated.
Acknowledgments
This study was supported by a grant from the National Cancer Institute (P01-CA108671) and the Ministero per la Ricerca Scientifica (RBNE0189JJ_003 and RBNE015P72_003) and a career development award from NYSTAR, NY.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Becker AJ, McCulloch CE, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 2.Jandl JH, Files NM, Barnett SB, Macdonald RA. Proliferative response of the spleen and liver to hemolysis. J. Exp. Med. 1965;122:299–326. doi: 10.1084/jem.122.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodsky I, Dennis LH, Kahn SB, Brady LW. Normal mouse erythropoiesis. I. The role of the spleen in mouse erythropoiesis. Cancer Res. 1966;26:198–201. [PubMed] [Google Scholar]
- 4.Bruce WR, McCulloch EA. The effect of erythropoietic stimulation on the hemopoietic colony-forming cells of mice. Blood. 1964;23:216–232. [PubMed] [Google Scholar]
- 5.Slayton WB, Georgelas A, Pierce LJ, et al. The spleen is a major site of megakaryopoiesis following transplantation of murine hematopoietic stem cells. Blood. 2002;100:3975–3982. doi: 10.1182/blood-2002-02-0490. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez M, Weissman IL, Pallavicini M, et al. Differential amplification of murine bipotent megakaryocytic/erythroid progenitor and precursor cells during recovery from acute and chronic erythroid stress. Stem Cells. 2006;24:337–348. doi: 10.1634/stemcells.2005-0023. [DOI] [PubMed] [Google Scholar]
- 7.Yin T, Li L. The stem cell niches in bone. J. Clin. Invest. 2006;116:1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 9.Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curry JL, Trentin JJ, Wolf N. Hemopoietic spleen colony studies. II. Erythropoiesis. J. Exp. Med. 1967;125:703–720. doi: 10.1084/jem.125.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yanai N, Satoh T, Obinata M. Endothelial cells create a hematopoietic inductive microenvironment preferential to erythropoiesis in the mouse spleen. Cell Struct. Funct. 1991;16:87–93. doi: 10.1247/csf.16.87. [DOI] [PubMed] [Google Scholar]
- 12.Derubeis AR, Mastrogiacomo M, Cancedda R, Quarto R. Osteogenic potential of rat spleen stromal cells. Eur. J. Cell Biol. 2003;82:175–181. doi: 10.1078/0171-9335-00300. [DOI] [PubMed] [Google Scholar]
- 13.Macias MP, Fitzpatrick LA, Brenneise I, et al. Expression of IL-5 alters bone metabolism and induces ossification of the spleen in transgenic mice. J. Clin. Invest. 2001;107:949–959. doi: 10.1172/JCI11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horowitz MC, Lorenzo JA. Immunologic regulation of bone development. Adv. Exp. Med. Biol. 2007;602:47–56. doi: 10.1007/978-0-387-72009-8_6. [DOI] [PubMed] [Google Scholar]
- 15.Nagayoshi K, Ohkawa H, Yorozu K, et al. Increased mobilization of c-kit+ Sca-1+ Lin- (KSL) cells and colony-forming units in spleen (CFU-S) following de novo formation of a stem cell niche depends on dynamic, but not stable, membranous ossification. J. Cell. Physiol. 2006;208:188–194. doi: 10.1002/jcp.20652. [DOI] [PubMed] [Google Scholar]
- 16.Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayack SR, Wagers AJ. Osteolineage niche cells initiate hematopoietic stem cell mobilization. Blood. 2008;112:519–531. doi: 10.1182/blood-2008-01-133710. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Visnjic D, Kalajzic Z, Rowe DW, et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 19.Vannucchi AM, Bianchi L, Cellai C, et al. Development of myelofibrosis in mice genetically impaired for GATA-1 expression (GATA-1(low) mice). Blood. 2002;100:1123–1232. doi: 10.1182/blood-2002-06-1913. [DOI] [PubMed] [Google Scholar]
- 20.Mallory FB. A contribution to staining methods. J. Exp. Med. 1900;5:15–20. doi: 10.1084/jem.5.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 2002;21:3368–3376. doi: 10.1038/sj.onc.1205326. [DOI] [PubMed] [Google Scholar]
- 22.Long MW, Williams N. Immature Megakaryocytes in the Mouse: Morphology and quantitation by acetylcholinesterase staining. Blood. 1981;58:1032–1039. [PubMed] [Google Scholar]
- 23.Lian JB, Stein GS. The developmental stages of osteoblast growth and differentiation exhibit selective responses of genes to growth factors (TGF beta 1) and hormones (vitamin D and glucocorticoids). J. Oral. Implantol. 1993;19:95–105. [PubMed] [Google Scholar]
- 24.Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr. Rev. 1993;14:424–442. doi: 10.1210/edrv-14-4-424. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, Zhou X, Kunkel G, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–27. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 26.Garimella R, Kacena MA, Tague SE, et al. Expression of bone morphogenetic proteins and their receptors in the bone marrow megakaryocytes of GATA-1(low) mice: a possible role in osteosclerosis. J. Histochem. Cytochem. 2007;55:745–752. doi: 10.1369/jhc.6A7164.2007. [DOI] [PubMed] [Google Scholar]
- 27.Kacena MA, Shivdasani RA, Wilson K, et al. Megakaryocyte-osteoblast interaction revealed in mice deficient in transcription factors GATA-1 and NF-E2. J. Bone Miner. Res. 2004;19:652–660. doi: 10.1359/JBMR.0301254. [DOI] [PubMed] [Google Scholar]
- 28.Purton LE, Scadden DT. Osteoclasts eat stem cells out of house and home. Nat. Med. 2006;12:610–611. doi: 10.1038/nm0606-610. [DOI] [PubMed] [Google Scholar]
- 29.Chang MK, Raggatt LJ, Alexander KA, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 30.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat. Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 31.Cervantes F, Mesa R, Barosi G. New and old treatment modalities in primary myelofibrosis. Cancer J. 2007;13:377–383. doi: 10.1097/PPO.0b013e31815a7c0a. [DOI] [PubMed] [Google Scholar]
- 32.Lataillade JJ, Pierre-Louis O, Hasselbalch HC, et al. Does primary myelofibrosis involve a defective stem cell niche? From concept to evidence. Blood. 2008;112:3026–3035. doi: 10.1182/blood-2008-06-158386. [DOI] [PubMed] [Google Scholar]