Abstract
Objectives
Estimate the probabilities, for Alzheimer's disease (AD) patients, of transitioning between stages of disease severity (mild, moderate, severe, dead) and care settings (community, institutional).
Methods
Data were compiled by the National Alzheimer Coordinating Center. The main analyses were limited to 3,852 patients who were >50 years old, diagnosed with possible/probable AD and had at least two center visits. A multinomial logistic model accounting for patient and center level correlation was used to calculate transition probabilities between stages of the Clinical Dementia Rating (CDR). Separately we calculated the probabilities of being institutionalized based on CDR stage. Both analyses controlled for baseline age, time between visits, sex, marital status, whether white, whether Hispanic and number of years of education.
Results
The annual probabilities of dying for patients in mild, moderate and severe health states were 5.5%, 21.5% and 48.0%, respectively, while the annual probabilities for institutionalization were 1.2%, 3.4% and 6.6%, respectively. The majority of mild and moderate patients remain in the same health state after one year, 77.4% and 50.1% respectively. Progressing patients are most likely to transition one stage, but 1.3% of mild patients become severe in one year. Some patients revert to lower severity stages, 7% from moderate to mild.
Conclusions
Transition probabilities to higher CDR stages and to institutionalization are lower than those published previously, but the probability of death is higher. These results are useful for understanding AD progression and can be used in simulation models to evaluate costs and compare new treatments or policies.
Keywords: Alzheimer disease, Natural history study, Progression, Transition probabilities
INTRODUCTION
Alzheimer disease is a progressive illness. The brain becomes increasingly damaged over time and symptoms become increasingly debilitating. Patients experience disease in their own way but understanding progression of disease allows patients and caregivers to prepare for future changes and the accompanying challenges.
From a policy perspective, disease progression informs upcoming changes in resource use and costs and allows comparisons to be made with new interventions. With the hope that new biologics or possibly other future treatments change the progression of AD it is important to understand the current disease progression to be able to assess the potential incremental benefit of new treatments.
Tacrine was the first cholinesterase inhibitor (CI) approved by the FDA in 1993 to treat cognitive symptoms [1] and was not widely prescribed. Since, treatment for AD has changed substantially with five new CI [2, 3] and other new treatments to ease behavioral symptoms [4, 5]. Current treatment plans also include management of comorbidities and counseling. Elderly patients use many new medications for other conditions that may change AD treatment or progression.
Neumann et al., calculated probabilities of transitioning between disease stages, and to institutionalization and death [6]. They used data from the Consortium to Establish a Registry for Alzheimer’ Disease (CERAD), a longitudinal study of 1145 dementia patients from 22 major medical centers in the United States from 1986 to 1995. Their results have been widely cited and used in modeling the cost-effectiveness of AD interventions [6–10]. However, these transition probabilities no longer represent patients receiving modern AD care.
The transition probabilities estimated in this analysis will allow researchers, practitioners, and policy makers to better understand AD progression, and will permit cost-effectiveness modelers to appropriately model progression for patients receiving the current standard of care.
MATERIAL AND METHODS
Participants
We used the Uniform Data Set (UDS) from the National Alzheimer Coordinating Center (NACC). The UDS contains demographic, clinical and specimen data from Alzheimer disease centers (ADCs) across the US. All ADCs enroll and follow patients annually with a standardized protocol and provide pooled data for research through the NACC. The UDS is not considered a population-based sample because of non-random enrollment of patients by participating ADCs. We limited data analysis to those patients who were 50 years of age or older. This study was approved by the University of Washington IRB.
Measures
Binomial variables were used to indicate whether a patient was institutionalized, white, married or Hispanic. Patients classified as institutionalized included those who resided in assisted living, boarding homes, adult family homes, skilled nursing facilities or nursing homes. We classified patients as married if they reported being married or living as married.
We measured education as the number of years of schooling. Baseline age was calculated as the difference in the birth year and the date of the initial visit. The day and month of birth and death were not included in the data set and assumed to be July 1. Thus, baseline ages are only approximate, as are time between visits.
We used the global Clinical Dementia Rating (CDR) scale to determine the stage of AD patients. CDR scores have been found to correlate well with other measures and to predict institutionalization and death [11]. CDR has also been correlated with patient quality of life and costs [12]. The lowest scores on the CDR (0.5 and 1) were combined and classified as mild as has been done previously [13]. Those that scored 2 or 3 were classified as moderate or severe, respectively. The previous CDR was calculated as the CDR stage at the last visit e.g. those classified as 'previously moderate' are those patients that were moderate at the previous visit.
Other measures were used to describe the population analyzed. The Mini Mental state examination (MMSE) is commonly used to measure cognitive impairment [14–16]. The Functional Activities Questionnaire (FAQ) is used in clinical situations for the assessment of functional deficit for the elderly [17]. To adjust for the number of tasks that were attempted by each individual we added the score for each task and divided by the number of tasks attempted. Individuals with full physical functioning score 0 while those dependent in each of the tasks attempted score 3. The Neuropsychiatric Inventory (NPI) was developed to assess the severity, frequency and caregiver distress of 12 different neuropsychiatric disturbances common in dementia [18–21]. Only the severity score of the NPI is contained within the UDS.
Pharmaceutical use in the 2 weeks prior to each ADC visit of 100 treatments of interest is collected in the NACC-UDS. Treatments of interest include all AD drugs, anti-psychotics and others, for a complete list see (https://www.alz.washington.edu/NONMEMBER/UDS/DOCS/VER2/ivpfillable.pdf).
Statistical Analyses
We first describe the population by CDR stages. We calculated the mean MMSE, NPI and FAQ scores by CDR stage to show the cognitive, behavioral and physical functioning differences. We also determined pharmaceutical use by diagnosis and CDR stage. To estimate the likelihood of pharmaceutical use we used logistic regression. We tested the association between pharmaceutical use in the last two weeks and AD diagnosis controlling for age, age-squared, sex, whether white, whether Hispanic and other cognitive impairment. For those pharmaceuticals that were significantly associated with AD diagnosis we calculated the percent of AD patients that used each treatment of interest by CDR stage.
We then calculated the association between previous and current CDR stages. We used multinomial logistic models with random effects to account for the individual and center level correlation. We adjusted for demographic characteristics of the patients including: sex, race, Hispanic, marital status, number of years of education and age. Since the duration between observations was not equal we adjusted for the exact time (days) between visits.
We also calculated the association between previous CDR stage and nursing home placement using logistic models and controlling for patient and center level correlations and the above mentioned demographics.
The coefficients from the multinomial and logistic regressions were used to derive the predicted probabilities [22, 23]. The point estimates and 95% confidence intervals were calculated using the delta method [24]. For the main transition probability tables we used the population means for each of the covariates. We estimated other transition probabilities for different populations to show the range of results.
We compared the transition probabilities we estimated to those estimated by Neumann et al., 2001. We calculated the percent of patients in each stage over time from the transition probabilities as was done by Neumann et al., 2001. We present 3 different scenarios, first we duplicate the results using the transition probabilities reported in Neumann et al., 2001, secondly we use the transition probabilities estimated from our statistical model using the mean patient characteristics from the NACC-UDS data, and third, we use the transition probabilities estimated from our statistical model using the patient characteristics from the CERAD population.
RESULTS
At the time of collection the UDS contained 17,736 patients from 32 different ADCs who had completed initial visits and met our inclusion criteria. Of these, 7,050 patients were diagnosed with possible or probable AD and 3,918 AD patients had more than one observation. Our main analyses were limited to 9,730 observations of 3,852 patients 50 years or older, with possible or probable AD, who had more than one observation and complete data for our covariates of interest.
From our descriptive analyses we find that patients are relatively mild and the majority are relatively new to the cohort (Table 1). Those mild, moderate or severe on the CDR had an average MMSE of 22.8, 14.6 or 5.3, respectively. Patients at higher stages on the CDR also had more physical and behavioral problems as measured by the FAQ and NPI (Supplementary Material 1).
Table 1.
Description of Population Analyzed
| Time in years between visits mean (sd) | 1.09(0.35) | n=5558 |
| Base Age mean (sd) | 77.31(9.12) | n=3852 |
| Years of Education mean (sd) | 14.24(3.74) | n=3852 |
| FAQ mean (sd) | 1.81(0.94) | n=5125 |
| NPI mean (sd) | 4.7(4.67) | n=5013 |
| MMSE | n=5023 | |
| MMSE score (sd) | 19.52(7.35) | |
| Physical Problem | 5.9% | |
| Cognitive/ Behavioral Problem | 57.5% | |
| Other | 30.3% | |
| Verbal Refusal | 6.4% | |
| Number of observations per patient | n=3852 | |
| Two | 55.5% | |
| Three | 36.5% | |
| Four | 7.9% | |
| Five | 0.1% | |
| Sex | ||
| Male | 47.8% | |
| Female | 52.2% | |
| Diagnosis | ||
| Probable AD | 79.9% | |
| Possible AD | 20.1% | |
| Residence at baseline | n=3852 | |
| Single Family | 83.3% | |
| Retirement community | 5.5% | |
| Assisted living/ boarding home/ adult family home | 5.6% | |
| Skilled nursing facility/ nursing home | 4.0% | |
| Other | 1.5% | |
| Unknown | 0.2% | |
| Living Situation at baseline | n=3852 | |
| Lives alone | 15.5% | |
| Lives with spouse or partner | 62.3% | |
| Lives with relative or friend | 13.0% | |
| Lives with group | 3.5% | |
| Other | 5.5% | |
| Unknown | 0.1% | |
| Marital status | ||
| Married | 65.0% | n=3852 |
| Widowed | 25.1% | |
| Divorced | 5.9% | |
| Separated | 0.6% | |
| Never married | 2.4% | |
| Living as married | 1.1% | |
| Other | 0.1% | |
| Unknown | 0.0% | |
| Race | n=3852 | |
| White | 82.6% | |
| Black or African American | 12.1% | |
| American Indian or Alaska Native | 0.3% | |
| Native Hawaiian or Other Pacific Islander | 0.1% | |
| Asian | 1.7% | |
| Other | 3.2% | |
| Unknown | 0.0% | |
| Hispanic | 7.4% | n=3852 |
Patients reported having taken an AD drug in the previous two weeks at 70.2% of their ADC visits (Supplementary Material 2).
The coefficients from the multinomial regressions represent the likelihood of transitioning into moderate, severe or dead states as compared to the mild state for a one unit increase in the independent variable of interest. In our analysis there are three odds ratios reported for each independent variable, one comparing the probability of being in a moderate state to a mild state, one comparing the probability of being in a severe state to a mild state and one comparing the probability of dying to being in a mild state. The OR 35.29 in the first row suggests that a patient currently in the moderate CDR stage is 35 times more likely to have been previously in a moderate health state at the last time of assessment rather than to have been previously in a mild health state. The coefficient for the previous CDR stage is positive and significant in all cases which suggests that being in a higher CDR stage at the previous visit is associated with being in a higher CDR stage or dead (Table 2). Demographic variables including age, whether white, whether Hispanic and years of education are significantly associated with dying. Older patients are 6% more likely to die than be in a mild state; white patients are 53% more likely than non-white patients to die than be in a mild state, all else equal.
Table 2.
The Odds of Each CDR Stage Compared to Mild
| CDR Stage Compared To Mild | Independent Variable | OR (95% CI) |
|---|---|---|
| Moderate | Previously moderate | 35.29 (26.40, 47.17) |
| Previously severe | 68.38 (9.00, 519.67) | |
| Time since last visit | 1.03 (0.81, 1.32) | |
| Age | 1.01 (1.00, 1.02) | |
| White | 0.94 (0.75, 1.18) | |
| Hispanic | 1.21 (0.88, 1.68) | |
| Female | 1.24 (1.04, 1.48) | |
| Years of education | 0.98 (0.96, 1.01) | |
| Married | 0.90 (0.74, 1.09) | |
| Constant | 0.14 (0.05, 0.35) | |
| Severe | Previously moderate | 183.01 (122.25, 273.97) |
| Previously severe | 15290.22 (2101.38, 111256.10) | |
| Time since last visit | 1.19 (0.85, 1.66) | |
| Age | 1.00 (0.99, 1.02) | |
| White | 0.79 (0.56, 1.11) | |
| Hispanic | 1.02 (0.63, 1.67) | |
| Female | 1.20 (0.90, 1.61) | |
| Years of education | 0.99 (0.95, 1.02) | |
| Married | 0.97 (0.70, 1.34) | |
| Constant | 0.01 (0.00, 0.06) | |
| Dead | Previously moderate | 43.39 (31.24, 60.27) |
| Previously severe | 3522.48 (490.14, 25314.69) | |
| Time since last visit | 0.45 (0.33, 0.62) | |
| Age | 1.06 (1.04, 1.07) | |
| White | 1.53 (1.11, 2.11) | |
| Hispanic | 0.37 (0.22, 0.62) | |
| Female | 0.87 (0.68, 1.10) | |
| Years of education | 0.96 (0.93, 0.99) | |
| Married | 0.92 (0.70, 1.19) | |
| Constant | 0.00 (0.00, 0.01) |
Number of Observations = 5558.
Number of Patients = 3852.
Number of NACC center = 32.
Log likelihood = −4271.24.
Variances and covariances of random effects.
level 2 (Patient) 4.36e-21 (1.66e-11).
level 3 (NACC center) 0.085 (0.037).
The results of our logistic regression suggest that previous CDR stage is strongly associated with whether a patient is institutionalized (Table 3).
Table 3.
The Odds of Institutionalization Compared to Community Living
| Institutionalization | OR (95% CI) |
|---|---|
| Previously moderate | 2.88 (1.69, 4.89) |
| Previously severe | 5.75 (2.65, 12.49) |
| Time since last visit | 2.23 (1.26, 3.93) |
| Age | 1.03 (1.00, 1.05) |
| White | 2.33 (1.28, 4.25) |
| Hispanic | 0.36 (0.16, 0.84) |
| Female | 0.73 (0.49, 1.1) |
| Years of education | 1.03 (0.97, 1.09) |
| Married | 0.24 (0.13, 0.42) |
| Constant | 0.00 (0.00, 0.01) |
Number of Observations = 4412*
Number of Patients = 3149.
Number of NACC center = 32.
Log likelihood = −707.18.
Variances and covariances of random effects.
level 2 (Patient) 1.53 (1.51).
level 3 (NACC center) 0.31 (0.16).
Limited from the main analysis to those who started in community living.
Age, whether white, whether Hispanic and marital status are also statistically significant factors and suggest that older, white, non-Hispanic, unmarried patients are more likely to be institutionalized, all else equal.
These transition probabilities suggest that patients are most likely to stay in the same stage year to year, 77% estimated to stay mild, 50% estimated to stay moderate and 49% estimated to stay severe. The next most likely transition is to the next higher stage of AD. There are also a small number of patients that transition to lower (i.e. less severe) stages; moderate patients are estimated to transition to mild in 7% of cases and about 3% of severe patients are estimated to regress. There were a small number of patients that transitioned from severe to mild but this result was not statistically significant (95% CI −0.002,0.006) The annual probabilities of dying for patients in mild, moderate and severe health states were 5.5%, 21.5% and 48.0%, respectively. The annual probabilities to institutionalization were 1.2% from mild, 3.4% from moderate and 6.6% from severe, although the transition from mild was not statistically significant (Table 4).
Table 4.
Annual Transition Probabilities between Severity Levels and to Death for the Average NACC-UDS Patient*
| t+1 | t+1 | |||||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Dead | Institutionalization | ||
| t | Mild (95%CI) |
0.774 (0.75, 0.798) |
0.158 (0.139, 0.176) | 0.013 (0.009, 0.017) | 0.055 (0.046, 0.064) | 0.012 (−0.003, 0.028) |
| Moderate (95%CI) |
0.07 (0.051, 0.089) |
0.501 (0.466, 0.536) | 0.214 (0.186, 0.243) | 0.215 (0.186, 0.244) | 0.034 (0.000, 0.069) |
|
| Severe (95%CI) |
0.002 (−0.002, 0.006) |
0.027 (0.013, 0.04) | 0.492 (0.447, 0.536) | 0.480 (0.435, 0.525) |
0.066 (0.005, 0.128) |
|
| Dead | 0 | 0 | 0 | 1 |
Time since last visit=1, Age=77.31, White=0.826, Hispanic=0.074, Female=0.522, Years of education=14.24 and Married=0.660.
The model estimates very different likelihoods of transitioning between 60 year old, white, Hispanic females with 22 years of education and married compared to 80 year old, non-white, non-Hispanic males with 12 years of education and unmarried (Supplementary Material 3). Transition probabilities for non-Hispanic whites, non-Hispanic blacks and Hispanics predict median time to death as 5.4 years, 6.6 years and 8.4 years, respectively.
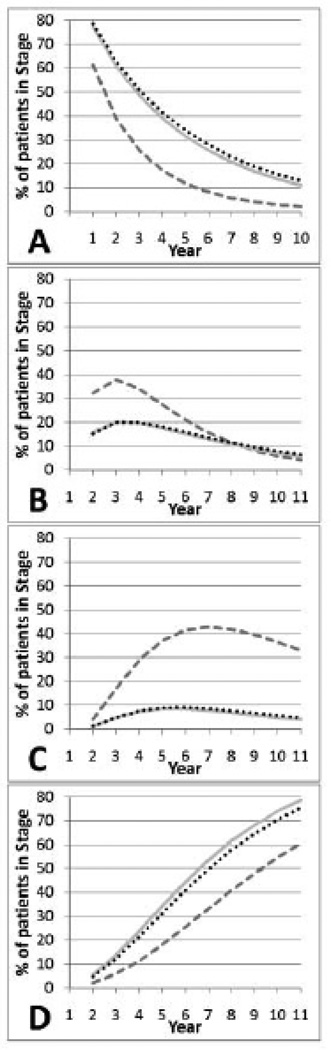
We compared the transition probabilities to those reported by Neumann et al., 2001 (Fig. 1). We found that patients were more likely to be mild or die using our data and model.
Fig. 1.
A comparison of CDR stage distribution over time between CERAD*, NACC-UDS† and the Model‡
 CERAD*
CERAD*  NACC-UDS†
NACC-UDS†  Model‡
Model‡
A - Mild Stage AD
B- Moderate Stage AD
C- Severe Stage AD
D - Dead
*CERAD - transitions as presented in Neumann et al.,
†NACC-UDS - transitions using NACC-UDS predictive model and population characteristics
‡Model - transitions using NACC-UDS predictive model and CERAD population characteristics
DISCUSSION
In this analysis we compute and report transition probabilities for AD patients. To do so we use a multivariate model and average population characteristics from the NACC-UDS. We also report regression coefficients that can be used to calculate transition probabilities for other populations of interest and we show how population characteristics change transition probabilities using an example of slow and fast transitioners.
We found that only the patients’ previous AD stage was significantly associated with their current AD stage. Age, gender, race and ethnicity do not significantly affect the likelihood of transitioning between AD stages in our analysis. We found that demographic factors and stage of disease severity are associated with dying and institutionalization.
The transition probabilities calculated in this analysis are useful for understanding AD progression and can be used in simulation models to evaluate costs and quality of life and compare new treatments or policies. Transition probabilities are commonly used by health economists to inform Markov models whereby costs and quality of life are assigned to health states and overall health and economic outcomes are estimated by tracking the time spent in different health states, as informed by the transition probabilities.
The NACC-UDS represents a large population from across the US. We found the AD population to be similar to other studies, although more educated [25, 26]. Our results are supported by others who have found that age, education, gender, race, ethnicity and marital status affect the mortality of AD patients [27–29]. In a systematic review of the effect of education on AD mortality Paradise et al., 2009 [30] conclude, contrary to our findings, that education does not lead to a difference in time to death after diagnosis.
The transition probabilities estimated suggest that a small number of patients improve their CDR score over time. This was also found by Neumann et al., who concluded these improvements were likely due to factors, such as variations in clinical assessment, the presence of depression or psychosis, or adjustments in medications that may mask actual disease stages. From this analysis we were unable to determine whether this result was measurement error or a true effect. A future analysis could consider this question more closely by also comparing other measures of AD.
Comparing our estimated transition probabilities to those in Neumann et al., part of the difference in the transitions is due to the difference in the patient characteristics between CERAD and NACC-UDS. To adjust for this difference we calculated transition probabilities using the patient characteristics of the CERAD population (Table 5).
Table 5.
Population Characteristics from NACC-UDS and CERAD
| Model Variables | NACC-UDS | CERAD |
|---|---|---|
| Base Age mean (sd) | 77.31(9.12) | 71.5 |
| Years of Education mean (sd) | 14.24(3.74) | 12.9 |
| Female | 52.2% | 53% |
| Married/ Living as married | 66.1% | Unknown |
| White | 82.6% | 96% |
| Hispanic | 7.4% | 1% |
Since the percent married was unknown we used the 66% as in NACC-UDS. Using the NACC-UDS predictive model and CERAD population characteristics the resulting transition probabilities were not greatly different from the predictive model using the NACC-UDS population characteristics but were slightly more similar to those of Neumann et al., Fig. (1). This is probably not a surprising result since the population characteristics used in the model had relatively small coefficients when estimating transitions between stages, but were larger and more significant when estimating institutionalization and death (Table 2). This result suggests that the difference between the two studies is something other than the population characteristics used in the model.
This difference in transition probabilities could be due to a shift in CDR staging over time. However, a recent study suggests that CDR drift is minimal and that the CDR demonstrates general stability for assessment of dementia over time [31]. The probability of death may differ due to the limited follow-up in the NACC-UDS and the high dropout rate in CERAD.
Modeling the NACC-UDS transition probabilities we found that 50% of the population was dead in approximately 5.5 years where as using the CERAD transitions it took approximately 8.5 years. The median time to death as predicted by the NACC-UDS transition probabilities are more similar to other published estimates than the CERAD transition probabilities. Helzner et al., 2008 [27] reported that median post-diagnosis survival for white non-Hispanics was 3.7 years, 4.8 years for black /African American and 7.6 years for Hispanics. We used our model to predict the probability of death in each of these populations and found a median survival for each group 1–2 years longer. This difference may be due to an under estimation of death in the NACC-UDS since death collection is ongoing and often delayed.
Patients transitioned to an institution more slowly in the NACC-UDS cohort than in CERAD. In following the assumption made by Neumann et al., we assumed that once a patient was institutionalized they stayed institutionalized although there were a small number of patients in the data set that transitioned back to the community. The low number of institutionalizations could be due to a less severe population or difficulty in following up with participants in nursing home. However we tried to control for the severity by including the previous CDR stage. Another difference between our study and Neumann et al., is the definition of institutionalization. CERAD did not gather information on entry into assisted living, but included only those who transitioned into nursing homes [32]. According to the National Center for Health Statistics nursing home residents have declined in the last 10 years. Of those 65 years or older the age-adjusted nursing home residents were 46.4 per 1000 in 1995 compared to 34.8 per 1000 in 2004.[33] There may be many reasons for this difference including financial and cultural changes or the benefit of new treatments. Mittelman et al., 2006 show that caregiver counseling and support group participation may delay nursing home admissions [34, 35]. Others have shown that for AD patients, anti-cholinesterase inhibitors and memantine may also delay time to nursing home placement [36, 37].
Many cost-effectiveness analyses have relied on the cost offsets of slowing nursing home progression due to treatment to show cost-effectiveness. These cost offsets will be less substantial if patients are being admitted less frequently. This result also suggests that it will be more important in the future to understand the effects of AD on family and caregivers as the responsibility shifts away from nursing homes.
The comparisons to Neumann et al., suggest that patients today are transitioning more slowly between disease stages and to institutionalization but dying more quickly. Given the difference in availability of pharmaceuticals between CERAD and NACC-UDS it may be tempting to suggest that the difference in transitions is due to treatment differences. However, although this is a possibility, we do not have sufficient information on patients' treatments to make any conclusions as to the cause of the change in transition probabilities. Although a large number of patients report using pharmaceutical treatments, many other counseling and support group interventions are available and not recorded in this data set. It might be the case that those who are more likely to take pharmaceutical treatments are also more likely to seek non-pharmaceutical care. It is also possible that transition probabilities are being affected by non-AD treatments or differences in life style such as healthy eating or less smoking. Our analysis could not control for these differences. The comparison of transition probabilities might also suggest that the benefits of current treatments are being offset by increased mortality. However we believe this is unlikely as our results are more in line with other studies of mortality in AD and believe we are more likely overestimating survival time for AD patients due to the time delay in reporting death.
We believe the current study can be improved by the addition of information on other non-pharmaceutical treatments for AD and with the addition of more longitudinal data. Fortunately the NACC-UDS is ongoing and will likely contain adequate follow-up to enrich the current findings within the next few years.
CONCLUSION
The transition probabilities reported here provide an estimation of progression through AD for those on current standard of care and may act as the comparator to new treatments. The reported regression results also allow researchers to calculate transition probabilities for specific populations. Even without new treatments in AD, these transition probabilities may be used in CEAs to determine the value of screening, different methods of diagnosis and other programs for AD patients and their caregivers. Future work may focus on estimating specific treatment effects on transition probabilities for AD patients using standard of care.
Supplementary Material
ACKNOWLEDGEMENTS
This research was completed in partial fulfillment of the dissertation of Eldon Spackman while a student at the University of Washington. The authors are grateful to Leslie Phillips for help in obtaining data from NACC-UDS. Eldon Spackman and Sean Sullivan had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the statistical analysis. Data collection was supported in part by National Institute on Aging (NIA) Grant (U01 AG016976) to the National Alzheimer's Coordinating Center. No funding was received for this study. Eldon Spackman had a pre-doctoral fellowship from the American Foundation of Pharmaceutical Education and has consulted with Genentech, Elan Pharmaceuticals and Bayer. Srikanth Kadiyala has no conflicts to disclose. Peter Neumann has consulted with Elan Pharmaceuticals. David Veenstra has consulted with Genentech. Sean Sullivan has consulted with Bayer and Elan Pharmaceuticals.
Footnotes
CONFLICT OF INTEREST
None declared.
- Mean cognition, physical functioning and behavior, as measured by the MMSE, FAQ and NPIQ, by CDR stage.
- Pharmaceutical use by CDR stage and the odds of using each pharmaceutical if a patient is diagnosed with AD, controlling for age, age-squared, sex, whether white, whether Hispanic, years of education, marital status and other cognitive impairment.
- A comparison of annual transition probabilities by selected demographic factors to show fast and slow transi-tioners.
REFERENCES
- 1.FDA-Approved Treatments for Alzheimer’s. 2007 Available from http://www.alz.org/national/documents/topicsheet_treatments.pdf.
- 2.Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimers Dement. 2008;4(2):110–133. doi: 10.1016/j.jalz.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 4.Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N Engl J Med. 2006;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 5.Sultzer DL, Davis SM, Tariot PN, Dagerman KS, Lebowitz BD, Lyketsos CG, et al. Clinical symptom responses to atypical antipsychotic medications in Alzheimer's disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am J Psychiatry. 2008;165(7):844–854. doi: 10.1176/appi.ajp.2008.07111779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neumann PJ, Hermann RC, Kuntz KM, Araki SS, Duff SB, Leon J, et al. Cost-effectiveness of donepezil in the treatment of mild or moderate Alzheimer's disease. Neurology. 1999;52(6):1138–1145. doi: 10.1212/wnl.52.6.1138. [DOI] [PubMed] [Google Scholar]
- 7.McMahon PM, Araki SS, Neumann PJ, Harris GJ, Gazelle GS. Cost-effectiveness of functional imaging tests in the diagnosis of Alzheimer disease. Radiology. 2000;217(1):58–68. doi: 10.1148/radiology.217.1.r00se1358. [DOI] [PubMed] [Google Scholar]
- 8.McMahon PM, Araki SS, Sandberg EA, Neumann PJ, Gazelle GS. Cost-effectiveness of PET in the diagnosis of Alzheimer disease. Radiology. 2003;228(2):515–522. doi: 10.1148/radiol.2282020915. [DOI] [PubMed] [Google Scholar]
- 9.Arrighi HM, Neumann PJ, Lieberburg IM, Townsend RJ. Lethality of alzheimer disease and its impact on nursing home placement. Alzheimer Dis Assoc Disord. 2009;24(1):90–95. doi: 10.1097/WAD.0b013e31819fe7d1. [DOI] [PubMed] [Google Scholar]
- 10.Claxton K, Neumann PJ, Araki S, Weinstein MC. Bayesian valueof-information analysisAn application to a policy model of Alzheimer's disease. Int J Technol Assess Health Care. 2001;17(1):38–55. doi: 10.1017/s0266462301104058. [DOI] [PubMed] [Google Scholar]
- 11.Dooneief G, Marder K, Tang MX, Stern Y. The Clinical Dementia Rating scale: community-based validation of "profound' and "terminal' stages. Neurology. 1996;46(6):1746–1749. doi: 10.1212/wnl.46.6.1746. [DOI] [PubMed] [Google Scholar]
- 12.Ekman M, Berg J, Wimo A, Jonsson L, McBurney C. Health utilities in mild cognitive impairment and dementia: a population study in Sweden. Int J Geriatr Psychiatry. 2007;22(7):649–655. doi: 10.1002/gps.1725. [DOI] [PubMed] [Google Scholar]
- 13.Neumann PJ, Araki SS, Arcelus A, Longo A, Papadopoulos G, Kosik KS, et al. Measuring Alzheimer's disease progression with transition probabilities: estimates from CERAD. Neurology. 2001;57(6):957–964. doi: 10.1212/wnl.57.6.957. [DOI] [PubMed] [Google Scholar]
- 14.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 15.Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. 2007;78(12):1298–1303. doi: 10.1136/jnnp.2006.109074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer RI, Kurosaki TT, Harrah CH, Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 18.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 19.Maidment ID, Fox CG, Boustani M, Rodriguez J, Brown RC, Katona CL. Efficacy of memantine on behavioral and psychological symptoms related to dementia: a systematic metaanalysis. Ann Pharmacother. 2008;42(1):32–38. doi: 10.1345/aph.1K372. [DOI] [PubMed] [Google Scholar]
- 20.Rocca P, Marino F, Montemagni C, Perrone D, Bogetto F. Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer's disease: preliminary findings from a naturalistic, retrospective study. Psychiatry Clin Neurosci. 2007;61(6):622–629. doi: 10.1111/j.1440-1819.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 21.Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA. 2003;289(2):210–216. doi: 10.1001/jama.289.2.210. [DOI] [PubMed] [Google Scholar]
- 22.Liao TF. In: Interpreting probability models logit, probit and other generalized linear models. Lewis-Beck MS, editor. Thousand Oaks: Sage Publications; 1994. [Google Scholar]
- 23.Pampel FC. Logistic regression : a primer. Thousand Oaks: Sage Publications; 2000. [Google Scholar]
- 24.Oehlert GW. A Note on the delta method. Am Stat. 1992;46(1):27–29. [Google Scholar]
- 25.Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). PartIClinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 27.Helzner EP, Scarmeas N, Cosentino S, Tang MX, Schupf N, Stern Y. Survival in Alzheimer disease: a multiethnic, population-based study of incident cases. Neurology. 2008;71(19):1489–1495. doi: 10.1212/01.wnl.0000334278.11022.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta KM, Yaffe K, Perez-Stable EJ, Stewart A, Barnes D, Kurland BF, et al. Race/ethnic differences in AD survival in US Alzheimer's Disease Centers. Neurology. 2008;70(14):1163–1170. doi: 10.1212/01.wnl.0000285287.99923.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steenland K, MacNeil J, Vega I, Levey A. Recent trends in Alzheimer disease mortality in the United States, 1999 to 2004. Alzheimer Dis Assoc Disord. 2009;23(2):165–170. doi: 10.1097/wad.0b013e3181902c3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paradise M, Cooper C, Livingston G. Systematic review of the effect of education on survival in Alzheimer's disease. Int Psychogeriatr. 2009;21(1):25–32. doi: 10.1017/S1041610208008053. [DOI] [PubMed] [Google Scholar]
- 31.Williams MM, Roe CM, Morris JC. Stability of the Clinical Dementia Rating, 1979–2007. Arch Neurol. 2009;66(6):773–777. doi: 10.1001/archneurol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson BL, Fillenbaum GG, Pieper CF, Heyman A. Home or nursing home: does place of residence affect longevity in patients with Alzheimer's disease? The experience of CERAD patients. Public Health Nurs. 2008;25(5):490–497. doi: 10.1111/j.1525-1446.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- 33.National Center for Health Statistics Health, United States. With Chartbook on Trends in the Health of Americans. Hyattsville, MD: 2007. [PubMed] [Google Scholar]
- 34.Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592–1599. doi: 10.1212/01.wnl.0000242727.81172.91. [DOI] [PubMed] [Google Scholar]
- 35.Brodaty H, Mittelman M, Gibson L, Seeher K, Burns A. The effects of counseling spouse caregivers of people with Alzheimer disease taking donepezil and of country of residence on rates of admission to nursing homes and mortality. Am J Geriatr Psychiatry. 2009;17(9):734–743. doi: 10.1097/jgp.0b013e3181a65187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldman HH, Pirttila T, Dartigues JF, Everitt B, Van Baelen B, Schwalen S, et al. Treatment with galantamine and time to nursing home placement in Alzheimer's disease patients with and without cerebrovascular disease. Int J Geriatr Psychiatry. 2009;24(5):479–488. doi: 10.1002/gps.2141. [DOI] [PubMed] [Google Scholar]
- 37.Lopez OL, Becker JT, Wahed AS, Saxton J, Sweet RA, Wolk DA, et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80(6):600–60s7. doi: 10.1136/jnnp.2008.158964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.