Abstract
Maintenance of skeletal muscle mass is contingent upon the dynamic equilibrium (fasted losses–fed gains) in protein turnover. Of all nutrients, the single amino acid leucine (Leu) possesses the most marked anabolic characteristics in acting as a trigger element for the initiation of protein synthesis. While the mechanisms by which Leu is ‘sensed’ have been the subject of great scrutiny, as a branched-chain amino acid, Leu can be catabolized within muscle, thus posing the possibility that metabolites of Leu could be involved in mediating the anabolic effect(s) of Leu. Our objective was to measure muscle protein anabolism in response to Leu and its metabolite HMB. Using [1,2-13C2]Leu and [2H5]phenylalanine tracers, and GC-MS/GC-C-IRMS we studied the effect of HMB or Leu alone on MPS (by tracer incorporation into myofibrils), and for HMB we also measured muscle proteolysis (by arteriovenous (A–V) dilution). Orally consumed 3.42 g free-acid (FA-HMB) HMB (providing 2.42 g of pure HMB) exhibited rapid bioavailability in plasma and muscle and, similarly to 3.42 g Leu, stimulated muscle protein synthesis (MPS; HMB +70%vs. Leu +110%). While HMB and Leu both increased anabolic signalling (mechanistic target of rapamycin; mTOR), this was more pronounced with Leu (i.e. p70S6K1 signalling ≤90 min vs. ≤30 min for HMB). HMB consumption also attenuated muscle protein breakdown (MPB; −57%) in an insulin-independent manner. We conclude that exogenous HMB induces acute muscle anabolism (increased MPS and reduced MPB) albeit perhaps via distinct, and/or additional mechanism(s) to Leu.
Key points
The branched-chain amino acid (BCAA) leucine acts as both a ‘trigger’ for the initiation of protein synthesis, and as a substrate for newly synthesized protein.
As a BCAA, leucine can be metabolized within skeletal muscle, leaving open the possibility that leucine metabolites might possess anabolic properties.
One metabolite in particular, β-hydroxy-β-methylbutyrate (HMB), has shown positive effects on lean body mass and strength following exercise, and in disease-related muscle wasting, yet its impact on acute human muscle protein turnover is undefined.
We report here that HMB stimulates muscle protein synthesis to a similar extent to leucine. HMB was also found to decrease muscle protein breakdown.
Our observation that HMB enhances muscle protein anabolism may partly (or wholly) underlie its pre-defined anabolic/anti-catabolic supplemental efficacy in humans.
Introduction
Postabsorptive periods are dominated by negative protein balance (muscle protein breakdown (MPB) exceeds muscle protein synthesis (MPS)) that can only be reversed by food intake. This ‘anabolic’ shift towards a positive protein balance is principally driven via a brief (∼2 h) but substantial (∼3-fold) postprandial elevation in MPS (Atherton et al. 2010a), with a small (∼50%) contribution from MPB depression (Wilkes et al. 2009). Consequently, it follows that adequate nutritional intake is crucial for maintenance of skeletal muscle mass.
Much work has been undertaken, over several years, in identifying anabolically active nutrients for skeletal muscle. Early experiments using stable isotope tracer techniques in humans showed that feeding a mixed macronutrient meal approximately doubled rates of MPS (Rennie et al. 1982). Subsequent studies infusing only amino acid (AA) mixtures confirmed the crucial role of essential AAs (EAAs) as the principal drivers of nutritionally stimulated MPS (Bennet et al. 1989). These studies were further refined where robust stimulation of MPS was observed with intravenous flooding doses of the single EAAs phenylalanine, valine and leucine (Leu; Smith et al. 1992, 1998). Since then a plethora of data, from both pre-clinical studies (Anthony et al. 2001; Suryawan et al. 2008) and human studies (Churchward-Venne et al. 2012) have established Leu as one of the most potent EAAs in terms of stimulating MPS. Indeed, in pre-clinical models, Leu is often used as a paradigm for stimulating muscle protein synthesis within the field of skeletal muscle protein metabolic research (Anthony et al. 2000a,b, 2001; Norton et al. 2012). Further underlining the integral role of Leu are studies showing that, of all the EAAs, Leu initiates the greatest ‘anabolic’ signalling responses – ostensibly through the mTORc1–p70S6K1 pathway (Atherton et al. 2010b). It is on this basis that Leu has been suggested as a direct modulator of MPS, in addition to the more obvious role as a substrate.
As a branched-chain amino acid (BCAA), Leu is transaminated in muscle by branched-chain aminotransferase (BCAT) to α-ketoisocaproate (α-KIC) before decarboxylation to isovaleryl-CoA by the mitochondrial enzyme branched-chain α-ketoacid dehydrogenase (BCKDH), then finally forming Acyl-CoA derivatives and entering the citric acid cycle. However, in the liver, an alternative metabolic fate for Leu has been shown wherein the cytosolic enzyme α-KIC dioxygenase (or 4-hydroxyphenylpyruvate dioxygenase (HPD); Van Koevering & Nissen, 1992) generates β-hydroxy-β-methylbutyrate (HMB) from Leu. This enzyme has been shown to be expressed in skeletal muscle, in addition to liver and kidney (Fig. 1), this leaves open the question as to whether metabolites of Leu could have direct anabolic effects within muscle. Although it has been scantily investigated and often trialled under poorly controlled experimental conditions for defining the effector (for example, providing Leu metabolites with other AAs), metabolites of Leu, HMB in particular, have been shown to enhance gains in muscle mass and strength. For example, on supplementation with 3 g of HMB during intense resistance exercise training (RET) for 3 weeks, healthy, untrained young men have shown a tendency to increase lean body mass (LBM) and increase strength when compared to a placebo-treated group (Nissen et al. 1996). Furthermore, a study in which elderly women received a supplement containing 2 g HMB plus 1.5 g of lysine and 5 g of arginine for 12 weeks revealed significantly greater strength gains and a trend for increased LBM in the supplemented group (Flakoll et al. 2004).
Figure 1. Gene expression of HPD.
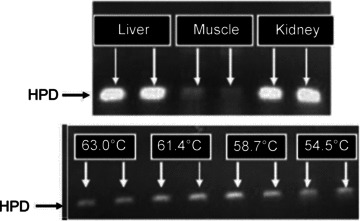
Upper panel, mouse tissues; lower panel, human skeletal muscle, with standard RT-PCR temperature gradient program shown for lower panel.
Indications from rodent and cell culture models are that HMB stimulates MPS in a similar fashion to Leu, that is through the mTOR–p70S6K1 pathway (Eley et al. 2007). Intriguingly, HMB can rescue depressions of MPS under both catabolic (e.g. elevated lipopolysaccharide (LPS), cytokines) and also under normal conditions through attenuating depressions in activity of the mTORc1 pathway (Eley et al. 2007). Moreover, HMB has also been shown to attenuate catabolism, reducing MPB via suppression of the ubiquitin-proteasome system (Eley et al. 2008), and by inhibition of myonuclear apoptosis by antagonizing mitochondrial-associated caspases (Hao et al. 2011). Some of these data have translated to human models of muscle catabolism such as: AIDS (Clark et al. 2000), cancer (May et al. 2002) and COPD (Hsieh et al. 2006). Collectively, these data support the notion that HMB could mediate its anabolic or anti-catabolic effects through acutely modulating muscle protein turnover.
To date, no study has investigated the possibility that HMB could represent an anabolic metabolite of Leu (responsible for acute effects on protein turnover). In this study we aimed to remedy that by studying the effects of HMB on human muscle protein turnover and compare this with Leu. Our hypotheses were: (i) HMB provision would acutely stimulate MPS (ii) HMB would stimulate MPS through mechanisms similar to its precursor, Leu, and (iii) HMB would also acutely depress MPB.
Methods
Ethical approval
Ethical approval was obtained from the University of Nottingham Medical School Ethics Committee and the Hamilton Health Sciences Research Ethics Board at McMaster University, with all studies being carried out in accordance with the Declaration of Helsinki. All volunteers were health-screened prior to participation, before providing written informed consent.
Subject characteristics and study design
Healthy young men (HMB study group: n= 8, mean age 22 ± 1 years, mean body mass index (BMI) 23 ± 1 kg m−2; Leu study group: n= 7, mean age 21 ± 0.3 years, BMI 25 ± 0.6 kg m−2), who were recreationally active but not involved in a formal training programme, were recruited to participate in the study. The two arms of the study were performed on different sites (Leu: McMaster University, Hamilton, Canada; HMB: University of Nottingham, UK), however both groups were recruited based on the same criteria, and did not differ significantly in age or BMI. The Leu and HMB studies differed slightly in that while both MPS and MPB were measured in the HMB study (Fig. 2A), only MPS was measured in the Leu feeding study (Fig. 2B). We chose not to measure MPB in the Leu study primarily due to the confounding factor of insulin secretion associated with Leu, since studies using large doses of AAs have failed to show an effect on MPB when insulin is clamped (Bennet et al. 1990; Greenhaff et al. 2008). Therefore we chose not to place the added burden of femoral lines on the subjects in the Leu study. Moreover, our principal focus was on delineating the anabolic effects of HMB, particularly in relation to MPS (the principal driver of nutrient-induced skeletal muscle anabolism). Volunteers were asked to refrain from heavy exercise for the 72 h before the study, and to fast from the night before the study, drinking only water ad libitum. On the morning of the study (∼08.30 h), subjects had an 18 g cannula inserted into the antecubital vein of one arm for tracer infusion, a retrograde 14 g cannula inserted to sample arterialized blood from the dorsal capillary bed of the hand (using the ‘hot hand’ method), and – in the HMB study only – had blood-sampling catheters inserted into the common femoral vein. A primed, continuous infusion (0.7 mg kg−1 prime, 1 mg kg h−1 continuous infusion) of [1,2-13C2]Leu tracer (99 Atoms%, Cambridge Isotopes Limited, Cambridge, MA, USA) – and in the HMB study [2H5]phenylalanine (0.3 mg kg−1 prime, 0.6 mg kg h−1 continuous infusion) – was started (at t=−2.5 h) after the first biopsy and maintained until the end of the study (+2.5 h). During the first 2.5 h period we gathered ‘baseline’ measurements. The subjects then drank either 3.42 g of a buffered and flavoured free-acid HMB solution (courtesy of Metabolic Technologies, Inc., Ames, IA, USA), which provided 2.42 g of HMB (solution: ∼70.8% HMB, 29.2% buffering and flavouring), or 3.42 g of L-Leu along with ∼400 ml of water, such that we were able to gather measures of the effects of HMB or Leu over the next 2.5 h. Precise timings of blood samples and muscle biopsies for the HMB and Leu studies can be seen in Fig. 2A and B. Muscle biopsies (∼200 mg) were taken from the vastus lateralis, under sterile conditions using a local anaesthetic (1% lidocaine).
Figure 2.
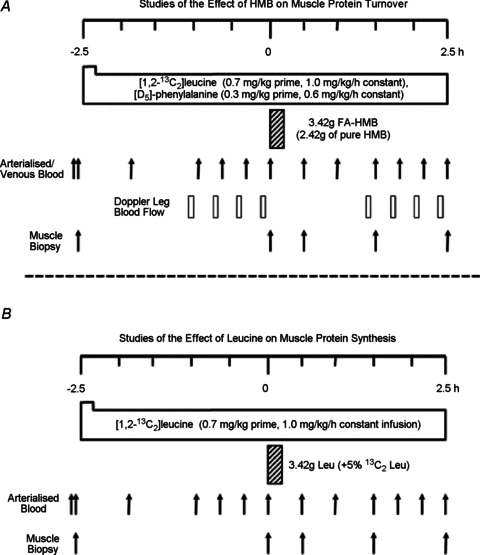
Study designs for assessing the anabolic effects of HMB (A) and Leu (B)
Analytical techniques
Blood samples were collected in anticoagulant pre-treated tubes and stored on ice before being separated at 2000 ×g for 20 min and the plasma supernatant decanted and stored at −80°C until later analyses. The muscle biopsies were washed in ice-cold phosphate-buffered saline, quickly separated from visible fat and connective tissue, flash-frozen in liquid nitrogen and stored for later analyses at –80°C. All analyses were performed at the University of Nottingham, with the exception of intracellular AA concentrations which were performed at McMaster University, Hamilton, and plasma/intramuscular HMB concentrations, which were performed by Metabolic Technologies Inc.
Plasma glucose and insulin concentrations
Postabsorptive plasma glucose concentrations were measured using an ILab 300 Plus Chemistry Analyser (Instrumentation Laboratory, Warrington, UK), and both fasted and postprandial plasma insulin concentrations were measured using undiluted samples on a high-sensitivity ELISA (DRG Instruments GmbH, Marburg, Germany).
Plasma and intracellular amino acid concentrations
For plasma samples, equal measures of plasma and sulfosalicyclic acid (containing 500 pmol μl−1 norleucine as an internal standard) were mixed and incubated at 4°C for 20 min, separated at 13,000 ×g for 5 min at −4°C and the supernatant was passed through a filter (0.22 μm pore size) before being analysed on a dedicated amino acid analyser (AAA; Biochrom 30: Biochrom, UK) utilizing a lithium buffer separation (Atherton et al. 2010a). All AA concentrations were determined by comparison to a standard sample and with use of the internal standard. Intracellular amino acids were prepared and analysed by HPLC as previously described (Glover et al. 2008).
Plasma and intramuscular HMB concentrations
Plasma HMB was analysed by gas chromatography–mass spectrometry (GC-MS) as previously described (Nissen et al. 1990). Muscle HMB was analysed using a modified approach based on the plasma analyses. Briefly, ∼100 mg of muscle tissue was transferred into a 12 × 75 mm plastic tube with internal standard. In addition, 0.5 ml of 3 n HCl and 0.5 ml of ultrapure H2O was added to the tube, and the contents were homogenized by using tissue homogenizer (Tissue Tearor, Biospec Products, Inc, Bartsville, OK, USA) for 1 min at 20,000 rev min−1. The homogenized contents were transferred to a 25 × 150 mm glass culture tube and extracted with ethyl ether. The HMB was then back-extracted into 0.1 m phosphate buffer, dried and analysed by GC-MS as for plasma.
Reverse-transcriptase polymerase chain reaction (RT-PCR) for HPD mRNA
Total RNA was isolated from human muscle or mouse liver kidney and skeletal muscle using TRI reagent (Sigma Aldrich, Poole, UK) and quantified with spectrophotometric analysis. cDNA was synthesized from 1 μg of total RNA using an iScript cDNA synthesis kit (Bio-Rad, Hemel Hempstead, UK). The cDNA (2 μl) was amplified using iQ SYBR Green Supermix (Bio-Rad, Hemel Hempstead, UK) and the following primers, designed for the human HPD (5′→3′): forward: CCCTGGAACAAAGAGATGGGCGAT; reverse: GATTTTGGCGCCCCGTTCCC and mouse: forward TCTGGTCCGTGGACGACACG; reverse TCCCTCAAGTGGCGGATTGCTG were used to determine gene expression of HPD. Amplification specificity was confirmed by running PCR temperature gradients with PCR products subject to melt-curve analysis and visualization on a 2% ethidium bromide-containing agarose gel to validate the product matched the predicted amplicon size.
Immunoblotting procedures
Immunoblotting was performed as previously described (Atherton et al. 2010b). Briefly, ∼30 mg of muscle was homogenized in ice-cold homogenization buffer (50 mm Tris-HCl (pH 7.4), 50 mm NaF, 10 mm β-glycerophosphate disodium salt, 1 mm EDTA, 1 mm EGTA, 1 mm activated Na3VO4 (all Sigma-Aldrich, Poole, UK)) and a complete protease inhibitor cocktail tablet (Roche, West Sussex, UK) at 10 μl μg−1 of tissue. Homogenates were rotated for 10 min and the supernatant collected by centrifugation at 13,000 × g for 5 min at 4°C. The supernatant (sarcoplasmic fraction) was used for immunoblot analysis and the pellet representing the myofibrillar fraction was stored at −80°C until subsequent MPS analysis (see Measurement of myofibrillar MPS). Sarcoplasmic protein concentrations were determined using a NanoDrop ND1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA) and adjusted to 1 μg μl−1 in 3× Laemmli buffer. Each sample was loaded onto pre-cast 12% Bis-Tris Criterion XT gels (BioRad, Hemel Hempstead, UK) at 10–15 μg per lane and separated electrophoretically at 200 V for 1 h. Proteins were then wet-transferred at 100 V for 1 h onto polyvinylidene difluoride (PVDF) membranes (0.22 μm pore size), blocked for 1 h in 2.5% skimmed milk in 1× Tris-buffered saline/Tween-20 (TBS-T), and then incubated in primary antibodies (1:2000 dilution in 2.5% BSA in TBS-T) rocking overnight at 4°C: AKTSer473, eEF2, eEF2Thr56, eIF2αSer51, Beclin 1, 4EBP1Thr37/46, eIF4E, GAPDH (New England Biolabs, Hertfordshire, UK), p70S6K1Thr389, Cathepsin L, Calpain 1, Caspase 3 (Abcam, Cambridge, UK), 4EBP1Ser65/Thr70 (Santa Cruz Biotechnology, Inc., Santa Cruz, US), MuRF1, Mafbx/Atrogin 1 (ECM Biosciences, Versailles, KY, USA). Membranes were subsequently washed 3 × 5 min in TBS-T, incubated in HRP-conjugated secondary antibody (New England Biolabs, Hertfordshire, UK; 1:2000 in 2.5% BSA in TBS-T) at room temperature for 1 h, before the last 3×5 min washes in TBS-T. Membranes were exposed to Chemiluminescent HRP Substrate (Millipore Corporation, Billerica, MA, USA) for 5 min and bands quantified by Chemidoc XRS (BioRad, Hertfordshire, UK). Software measures were taken to prevent pixel saturation and protein loading anomalies were corrected to total GAPDH, total eIF4E or Coomassie total protein.
Plasma AA labelling
To determine labelling (atom percent excess; APE) and/or concentrations of arterialized venous and venous Leu, phenylalanine, and α-ketoisocaproate (α-KIC): briefly, plasma protein was precipitated using 100% ethanol and the quinoxalinol KIC derivative formed then extracted into ethyl acetate, dried down, and derivatized to its t-butyldimethylsilyl (tBDMS) -quinoxalinol form. The aqueous layer, containing the amino acids was dried down and the tBDMS derivative of Leu and phenylalanine formed. Enrichments were then measured by GC-MS by selected ion-monitoring (SIM), m/z 259, 261 for α-KIC, 302, 304 for Leu and 234, 236, 239 for phenylalanine. Concentrations for plasma phenylalanine were determined using 2H2 phenylalanine, with reference to a standard curve.
Measurement of myofibrillar MPS
The pellet from the immunoblot preparation was washed three times with homogenization buffer (see Immunoblotting procedures) and 0.3 m NaOH was added in order to facilitate the separation of the soluble myofibrillar fraction from the insoluble collagen fraction by subsequent centrifugation. The myofibrillar fraction was then removed and precipitated using 1 m perchloric acid (PCA) and pelleted by centrifugation. The myofibrillar pellet was then washed twice with 70% ethanol and the protein-bound AAs were released by acid hydrolysis using 0.1 m HCl and 1 ml of Dowex ion-exchange resin (50W-X8-200) heated overnight at 110°C. AAs were further purified by ion-exchange chromatography on Dowex H+ resin columns before being eluted with NH4OH and derivatized to their N-acetyl-N-propyl esters. The samples were analysed using capillary gas chromatography–combustion–isotope-ratio mass spectrometry (GC-C-IRMS) on a Delta Plus XL (Thermo Fisher Scientific, Hemel Hempstead, UK) using our standard techniques (Kumar et al. 2009). The fractional synthetic rate (FSR) of the myofibrillar proteins was calculated using a standard precursor–product paradigm:
where δEm is the change in the [1,2-13C2]Leu enrichment in atoms per excess (APE) between subsequent biopsies, separated by the time period (t), and Ep is the mean enrichment over the same time period (t) of the precursor for protein synthesis, i.e. venous plasma α-KIC was used as a proxy for leucyl-tRNA, the immediate precursor for protein synthesis (Watt et al. 1991).
Measurement of MPB
Leg muscle protein flux (muscle protein breakdown, MPB) was calculated as previously described by arteriovenous (A–V) dilution of the [2H5]-phenylalanine tracer (Bennet et al. 1990):
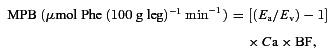 |
Where Ea and Ev are the steady state [2H5]phenylalanine enrichment values of arterialized and venous samples, respectively, Ca is the mean [2H5]phenylalanine concentration in arterialized plasma, and BF is arterial blood flow in ml leg−1, adjusted for plasma using the haematocrit.
Statistical analyses
Descriptive statistics were produced for all data sets and checked for normal distribution using a Kolgomorov-Smirnov test. Normal distribution was accepted if P > 0.05. Following correction for loading anomalies, protein phosphorylation data were normalized to the fasted time point, which was itself normalized to a mean of 1 to retain data variance. Graphs are presented as means ± SEM with differences detected with a two-way measures analysis of variance with Bonferroni correction, or on a pairwise t test basis for HMB MPB data (Graph Pad, Version 5, La Jolla, San Diego, CA, USA). Statistically significant results were designated with notations according to the figure legends where the statistical tests indicated that the threshold of P < 0.05 was passed.
Results
Effects of oral HMB or Leu on plasma HMB, Leu and insulin concentrations
With HMB ingestion, fasted plasma HMB concentrations of 5.1 ± 1 μm increased to 408 ± 27 μm (Fig. 3A) by 30 min and remained significantly elevated over the basal value by the end of the study (275 ± 12 μm 150 min post HMB consumption; P < 0.001). With Leu ingestion, HMB concentrations in plasma were unaltered until 150 min whereby HMB concentrations had risen modestly from 3.2 ± 0.6 to 10.3 ± 3.5 μm (P < 0.05). With HMB ingestion, plasma Leu concentrations were unaltered throughout (0 min 146 ± 3 versus 142 ± 3 μm 30–150 min). After Leu ingestion, plasma Leu concentrations (fasted: 142 ± 5 μm) increased by 30 min (495 ± 28 μm; P < 0.01) and remained significantly elevated at 60 min (375 ± 32 μm; P < 0.01) before returning to values not statistically different from those of the fasted condition (Fig. 3B). Plasma insulin concentration remained unaltered in response to HMB consumption (post HMB study mean 5.9 ± 1 mU l−1; Fig. 3C). However, in response to Leu, plasma insulin had increased from baseline (6 ± 2 mU l−1) by 30 min (10 ± 2 mU l−1; P < 0.05) thereafter returning to values not different from baseline (Fig. 3C).
Figure 3. Plasma HMB (A), Leu (B) and insulin (C) in response to oral HMB (open circle) or Leu (filled circle) consumption.
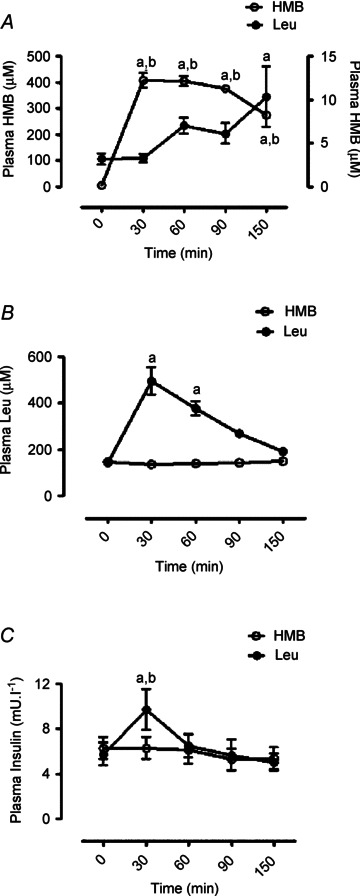
Left y-axis represents scale for HMB group, right y-axis represents scale for Leu group. Letters indicate statistical significance (P < 0.05): a = different from respective basal; b = different between groups at equivalent time-point. Data are presented as means ± SEM.
Effects of oral HMB or Leu on intramuscular HMB, Leu and EAA concentrations
Fasted concentrations of intramuscular HMB (7 ± 3 μmol l−1) were increased after oral HMB consumption (150 min: 96 ± 13 μmol l−1; P < 0.01) but were not detectably altered after Leu consumption (Fig. 4A). While oral HMB consumption did not affect intramuscular concentrations of Leu or total EAAs, Leu consumption increased intramuscular Leu concentrations from 120 μmol l−1 at basal to 167 ± 8 μmol l−1 at 150 min (P < 0.05; Fig. 4B) without significantly increasing summed EAA concentrations.
Figure 4. Intramuscular concentrations of HMB (A), EAA (B) and Leu (C) in response to oral HMB (open circle) or Leu (filled circle) consumption.
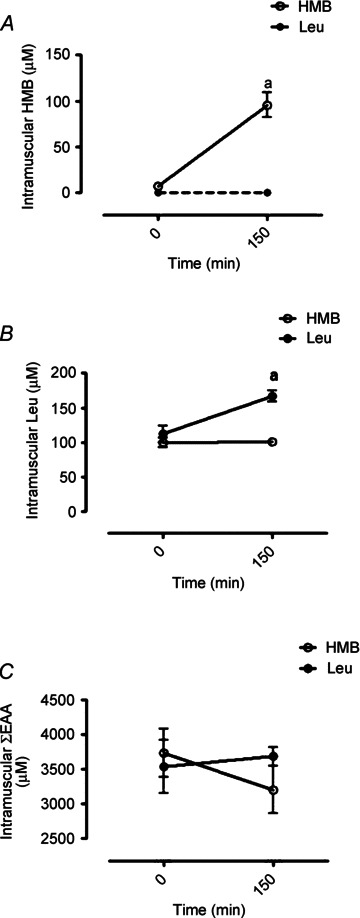
Dashed line in A indicates intramuscular HMB concentration was below detection limit. Letters indicate statistical significance (P < 0.05): a = different from respective basal; b = different between groups at equivalent time point. Data are presented as means ± SEM.
The effects of oral HMB on MPS and MPB, and of oral Leu on MPS
The fractional synthesis (FSR) representing MPS of myofibrillar proteins (Fig. 5A) was increased, from fasted values of 0.043 ± 0.004 to 0.073 ± 0.01% h−1, 150 min after the HMB feed (∼70%P < 0.05). Leu provision increased myofibrillar FSR (Fig. 4A) from a basal rate of 0.042 ± 0.007 to 0.088 ± 0.005% h−1 over the 2.5 h post-feeding period (∼110%; P < 0.01). The effects of HMB and Leu on MPS were not statistically different (P= 0.12). Over the same time period, HMB consumption reduced leg proteolysis (an index of MPB; Fig. 5B) from 12 ± 4 to 5 ± 1 μmol Phe l−1.min−1 (∼57%, P < 0.05). To elucidate a potential mechanism regulating this reduced leg proteolysis with HMB, the abundance of a number of molecular markers of proteolytic pathways such as: MuRF1 and Mafbx/Atrogin1 (ubiquitin-proteasomal), Beclin 1 (autophagy), Cathepsin L (lysosomal), Calpain 1 (calcium dependent protease) were measured using Western blotting. There were no changes observed in any of these targets (Fig. 6), with no active forms of Caspase 3 detected either (data not shown).
Figure 5. Myofibrillar FSR in response to oral HMB (open circle) or Leu (filled circle) consumption (A) and leg proteolysis in response to HMB consumption (B).
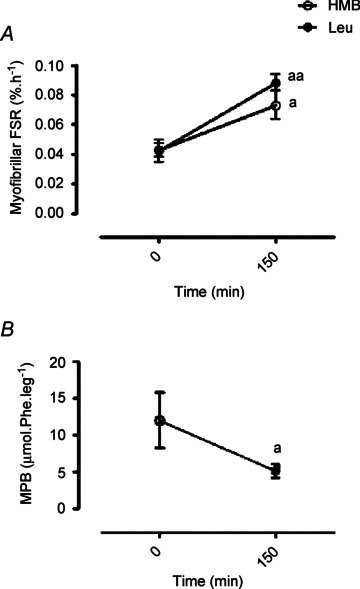
Letters indicate statistical significance (P < 0.05): a = different from respective basal (a P < 0.05; aa P < 0.01). Data are presented as means ± SEM.
Figure 6. Beclin 1 (A), Mafbx (B), MuRF 1 (C), Calpain 1 (D), Cathepsin L (E) in response to oral HMB consumption.
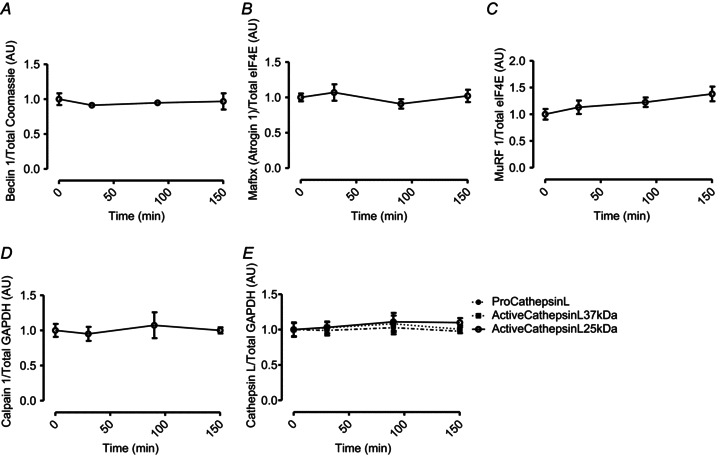
Data are presented as mean ± SEM.
The effects of HMB and Leu on mTORC1 signalling
Phosphorylation of AKTSer473 did not alter in response to HMB. In contrast, Leu consumption resulted in increased concentrations of AKTSer473 (∼36%, P < 0.05) 30 min after consumption (Fig. 7A). The phosphorylation of p70S6K1 increased similarly in both HMB (∼56%) and Leu (∼45%, P < 0.05) groups at 30 min (Fig. 7B). However, by the 90 min time point increased p70S6K1 phosphorylation was maintained (∼71%) only in the Leu group. After 30 min ingestion of HMB and Leu, there was a modest increase in 4EBP1Ser65/Thr70 phosphorylation (∼27% and ∼18%, respectively; P < 0.05), but not for phosphorylation at its Thr37/46 sites (Fig. 7D). As with p70S6K1, increased phosphorylation of 4EBP1Ser65/Thr70 was maintained with Leu at 90 min (∼19%; P < 0.05) but not with HMB (Fig. 7C). Phosphorylation of eIF2αSer51 and eEF2Thr56 did not change in either the HMB or Leu groups (Fig. 7E and F).
Figure 7. AKTSer473 (A), p70S6K1Thr389 (B), 4EBP1Ser65/Thr70 (C), 4EBP1Thr37/46 (D), eIF2αSer51 (E), eEF2Thr56 (F) in response to oral HMB (open circle) or Leu (filled circle) consumption.
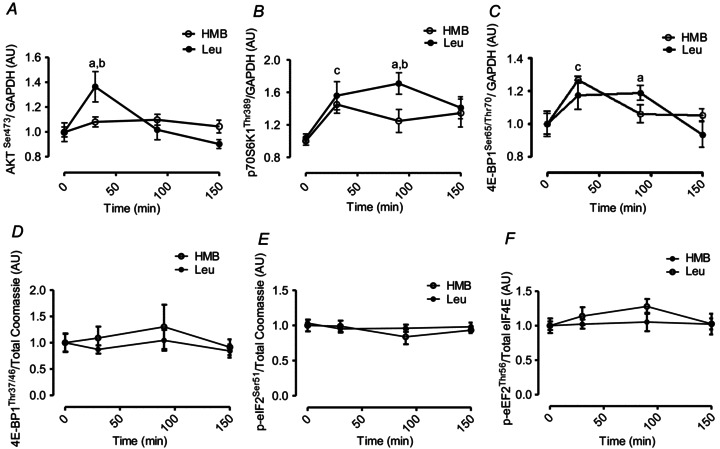
Statistical notations: a = different from respective basal; (P < 0.05), b = different between groups at equivalent time-point (P < 0.05), c = different from respective basals under both conditions (P < 0.05). Data are presented as means ± SEM.
Discussion
Nutrient-mediated increases in MPS preserve the net muscle protein equilibrium (fasted losses vs. fed gains) to ensure muscle mass remains constant. Much of this effect has been attributed to the single EAA Leu (Kimball & Jefferson, 2006). Although the majority of focus has been on identifying proximal signalling mechanisms by which BCAA, and particularly Leu stimulated MPS, Leu is also able to undergo catabolism in muscle and consequently we were interested in exploring the possibility that Leu metabolites could possess anabolic attributes. With previous evidence in the literature suggesting HMB may possess anabolic potential, we consequently focused on the role of HMB.
We report that oral consumption of HMB in free-acid form (FA-HMB), rapidly elevated plasma and intramuscular HMB bioavailability from fasting concentrations. We chose FA-HMB over the more commonly used calcium-HMB (Ca-HMB) principally because of the more favourable bioavailability kinetics (Fuller & Sharp, 2011). When comparing the effects of HMB vs. Leu on MPS, we found a robust stimulation (HMB ∼70%vs. Leu ∼110%). To place this size of response to HMB or Leu in context with a more substantial feeding regime, our previous study of feeding 48 g whey protein to young men (Atherton et al. 2010a) resulted in ∼2.5-fold increases in MPS over a 1.5 h period post-feed, before MPS returned to baseline over the next period measured (1.5–2.5 h). If this response were to be directly compared with this present Leu and HMB work, made over a 2.5 h measurement period, then overall prandial protein accretion via increases in MPS would be roughly similar (note that MPS is calculated as incorporation/time). These facts are striking and imply that it is possible to robustly increase MPS responses to nutrition by supplying complex mixtures of EAAs but also Leu, and even HMB, alone, at least until EAA and NEAA substrate depletion becomes rate limiting for MPS (Churchward-Venne et al. 2012). Notably, there was tendency for the rates of MPS to be greater with Leu vs. those seen with HMB (P= 0.12); these data imply that HMB is slightly less effective in stimulating MPS than Leu, at least at presently administered doses.
Due to its insulin secretagogue properties (Zhou et al. 2010), Leu, as expected, induced a transient spike in plasma insulin at 30 min after consumption. On this basis, some might attribute a portion of the Leu-mediated increases in MPS to insulin. However, when AAs are infused with insulin clamped at post-absorptive concentrations (∼5 μU ml−1) the MPS response is not dampened (Greenhaff et al. 2008), suggesting that insulin is at best permissive for the stimulation of MPS. Moreover, HMB did not affect insulin secretion despite generating comparable protein synthetic responses to that of Leu. Therefore, as we have identified that HMB, unlike Leu, is not an insulin secretagogue, it is unlikely that insulin is responsible for the changes in MPS we observe with Leu.
Ingestion of Leu induced an ‘anabolic’ signalling response in AKT, p70S6K1 and 4EBP1 (at least in as much as phosphorylation indicates activation of these proteins) that was of a lesser amplitude (∼71%vs. ∼112% increase at 90 min post-feeding) and duration than in our previous study involving the feeding 48 g of whey protein isolate (Atherton et al. 2010a). The lesser response may have been due to a lower dose of Leu (3.42 g in the present study vs. 6 g in the whey protein study) or possibly that other EAAs are required to maximize signalling through mTORc1 (Atherton et al. 2010b; Churchward-Venne et al. 2012). Regardless, this raises further caution in terms of interpreting changes in mTORc1 signalling amplitude as a reliable proxy of MPS (Greenhaff et al. 2008).
HMB-induced anabolic signalling was even less distinct than that seen with Leu since there was no modulation of AKT and a reduced duration of p70S6K1 and 4EBP1 signalling (∼30 min vs. 30–90 min after Leu) after HMB consumption. The lack of AKT modulation with HMB was almost certainly due to the fact that HMB, unlike Leu, did not promote insulin secretion (Fig. 3C). This is because EAAs signal through distinct proximal insulin receptor response elements such as the Rag GTPases (Sancak et al. 2008) or leucyl tRNA synthetase (Han et al. 2012), whereas insulin signals though the canonical insulin receptor substrate–phosphoinositide-3-kinase–AKT (IRS-1-PI3K-AKT) signalling pathway. The stimulation of MPS through mTORc1-signalling following HMB exposure is in agreement with pre-clinical studies (Eley et al. 2008). However, the reduced magnitude of signalling compared to Leu suggests, at least in context of the quantities provided, that Leu is more a potent signalling agent than HMB. One possibility is that multiple metabolites of Leu are capable of stimulating MPS such as α-KIC (Escobar et al. 2010). Nonetheless, as the overall MPS response was similar, this cellular signalling distinction did not translate into statistically distinguishable anabolic effects in our primary outcome measure of MPS.
Furthermore, there was clear divergence in the amplitude of phosphorylation for 4EBP1 (at Thr37/46 and Ser65/Thr70) and p70S6K (Thr389) in response to both Leu and HMB, with the latter showing more pronounced and sustained phosphorylation. It has been shown that the phosphorylation of 4EBP1 is a two-step process: while mTOR phosphorylation at Thr37 and Thr46 does not prevent the binding of 4EBP1 to eIF4E, it is thought to prime 4EBP1 for subsequent phosphorylation at Ser65/Thr70 (Gingras et al. 1999) which then promotes eIF4F complex assembly. While we were unable to detect such ‘priming’ (Thr37/46) phosphorylation on 4EBP1 after Leu or HMB, we did detect significant increases on Ser65/Thr70, which perhaps mediated increases in MPS though assembling eIF4F and thus promoting cap-dependent translation. Also, since previous work by us and others revealed discrepancies in the amplitude of 4EBP1 and p70S6K phosphorylation following resistance exercise (Kumar et al. 2009; Holm et al. 2010) and nutrition (Fujita et al. 2007; i.e. phosphorylation of p70S6K being more pronounced than 4EBP1), this is perhaps why p70S6K, rather than 4EBP1, is most commonly adopted as a proxy for mTOR signalling.
Interestingly, although orally supplied HMB produced no increase in plasma insulin, it caused a depression in MPB (−57%). Normally, postprandial decreases in MPB (of ∼50%) are attributed to the nitrogen-sparing effects of insulin since clamping insulin at post-absorptive concentrations (5 μU ml−1) while continuously infusing AAs (18 g h−1) did not suppress MPB (Greenhaff et al. 2008), which is why we chose not to measure MPB in the Leu group, due to an anticipated hyperinsulinaemia (Fig. 3C). Thus, HMB reduces MPB in a fashion similar to, but independent of, insulin. These findings are in-line with reports of the anti-catabolic effects of HMB suppressing MPB in pre-clinical models, via attenuating proteasomal-mediated proteolysis in response to LPS (Eley et al. 2008). In attempt to determine the regulatory mechanism behind this insulin-independent decrease in MPB with HMB, a number of molecular targets associated with different proteolytic systems (the ‘atrogenes’, Beclin 1, Calpain 1, Cathepsin L and Caspase 3) were examined. However there were no changes in the abundance of protein observed any of these and we were also unable to detect any changes in post-translational indicators of proteolytic activity, i.e. autolytic cleavage of Calpain 1 or detection of active (cleaved) forms of Caspase 3. These findings are in agreement with a past study of ours showing that acute depressions in MPB, under insulin-clamped insulin conditions, yielded no associations to the abundance of protein or mRNA of several of these proteolytic ‘marker’ genes (Greenhaff et al. 2008). Moreover, while, for example, the atrogenes (MuRF-1 and Mafbx) can robustly inform upon chronic scenarios of protein catabolism (sepsis, burns, trauma; Murton et al. 2008) associated with skeletal muscle atrophy, it would appear they are not (measurably) sensitive to acute episodic depressions in MPB with nutrition. Therefore, until protein kinases/phosphorylation sites of signalling pathways that inform on acute/nutrition-mediated depressions in MPB are identified, linking single or multiple MPB-related pathways defining the mechanisms behind the acute anti-catabolic effects of HMB/insulin remains intractable. Nonetheless, our observations set HMB apart from hyperaminoacidaemia which could not suppress MPB in the absence of hyperinsulinaemia (Greenhaff et al. 2008).
Lastly, we would like to acknowledge a number of potential study limitations. The groups were studied in different locations: HMB at the University of Nottingham, UK and Leu at McMaster University, Canada. Nonetheless, the protocol used and recruitment criteria were identical between both groups, with both labs involved having extensive experience of conducting such work and all components of analysis were completed at the University of Nottingham (with the exception of HMB and intracellular AA concentrations). However, we are not aware of data to suggest that Europeans and North Americans respond differently to nutrition (e.g. leucine); indeed the MPS responses to Leu herein are identical to those we have published previously (Smith et al. 1992). We did not control for dietary protein intake or habitual activity. However, all volunteers were confirmed as only recreationally active and not in formal training regimes and we are unaware of data to show that different habitual protein intake modulates acute anabolic responses to Leu. Furthermore, it was not possible from the present study to determine a mechanism for the observed HMB reduction in MPB, despite measuring the abundance/post-translation modifications of caspase, calpain, autophagy and proteasomal pathway gene markers. Whilst we acknowledge these potential limitations, our work depicts for the first time a comparison of the acute effects of consumption of Leu and its metabolite HMB in young men. Consumption of small amounts (∼2–3 g) of either Leu or its metabolite HMB resulted in the acute increase of MPS to a degree comparable to that seen after a mixed meal, with HMB also suppressing MPB.
Acknowledgments
Metabolic Technologies Inc. supplied the HMB on a collaborative basis and undertook the HMB plasma and intramuscular analyses, but were blinded to the sample identities. P.J.A. was a designated RCUK Fellow. Work completed in the UK was funded by grants from the Royal Society (RG2010/R2) and the University of Nottingham. D.H., H.C. and N.J.S. were supported by the US NIH (AR-054342). D.J.W. and N.J.S. were supported by the MRC (G0801271). Portions of work completed by T.A.C.-V. and L.B. were supported by a grant from the Canadian National Science and Engineering Research Council (NSERC) of Canada to S.M.P. We gratefully acknowledge the work of T. Rerecich in assisting with lab analyses.
Glossary
- 4EBP1
eukaryotic initiation factor 4E binding protein 1
- α-KIC
α-ketoisocaproate
- AA
amino acid
- AKT
protein kinase B
- APE
atom percent excess
- BCAA
branched-chain amino acid
- BCAT
branched-chain aminotransferase
- BCKDH
branched-chain α-ketoacid dehydrogenase
- Ca-HMB
calcium β-hydroxy-β-methylbutyrate
- EAA
essential amino acid
- FA-HMB
free acid β-hydroxy-β-methylbutyrate
- FSR
fractional synthetic rate
- GC-MS
gas chromatography–mass spectrometry
- GC-C-IRMS
gas chromatography–combustion–isotope ratio mass spectrometry
- HMB
β-hydroxy-β-methylbutyrate
- HPD
4-hydroxyphenylpyruvate dioxygenase
- LBM,
lean body mass
- MPB
muscle protein breakdown
- MPS
muscle protein synthesis
- mTOR
mechanistic target of rapamycin (previously known as: mammalian target of rapamycin)
- Leu
leucine
- p70S6K1
70 kDa ribosomal protein S6 kinase 1
Author contributions
P.J.A., K.S., N.J.S., J.W. and P.L. conceived and designed the experiments. T.H., D.S.H., J.W., L.B., S.M.P., L.B. and T.A.C.-V. performed all data collection. D.J.W., D.S.H., B.E.P., H.C., T.E., L.B., T.A.C.-V. and J.A.R. performed all data analysis and interpretation. D.J.W., D.S.H., P.J.A. and K.S. drafted the manuscript. All authors contributed to the revisions of the manuscript and approved the final version of the manuscript.
References
- Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr. 2001;131:856S–860S. doi: 10.1093/jn/131.3.856S. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000a;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000b;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010a;92:1080–1088. doi: 10.3945/ajcn.2010.29819. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino acids. 2010b;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Rennie MJ. The effect of amino acid infusion on leg protein turnover assessed by L-[15N]phenylalanine and L-[1-13C]leucine exchange. Eur J Clin Invest. 1990;20:41–50. doi: 10.1111/j.1365-2362.1990.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Bennet WM, Connacher AA, Scrimgeour CM, Smith K, Rennie MJ. Increase in anterior tibialis muscle protein synthesis in healthy man during mixed amino acid infusion: studies of incorporation of [1-13C]leucine. Clin Sci (Lond) 1989;76:447–454. doi: 10.1042/cs0760447. [DOI] [PubMed] [Google Scholar]
- Churchward-Venne TA, Burd NA, Mitchell CJ, West DWD, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590:2751–2765. doi: 10.1113/jphysiol.2012.228833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RH, Feleke G, Din M, Yasmin T, Singh G, Khan FA, Rathmacher JA. Nutritional treatment for acquired immunodeficiency virus-associated wasting using beta-hydroxy beta-methylbutyrate, glutamine, and arginine: a randomized, double-blind, placebo-controlled study. JPEN J Parenter Enteral Nutr. 2000;24:133–139. doi: 10.1177/0148607100024003133. [DOI] [PubMed] [Google Scholar]
- Eley HL, Russell ST, Baxter JH, Mukerji P, Tisdale MJ. Signaling pathways initiated by beta-hydroxy-beta-methylbutyrate to attenuate the depression of protein synthesis in skeletal muscle in response to cachectic stimuli. Am J Physiol Endocrinol Metab. 2007;293:E923–E931. doi: 10.1152/ajpendo.00314.2007. [DOI] [PubMed] [Google Scholar]
- Eley HL, Russell ST, Tisdale MJ. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-alpha and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab. 2008;295:E1417–E1426. doi: 10.1152/ajpendo.90567.2008. [DOI] [PubMed] [Google Scholar]
- Escobar J, Frank JW, Suryawan A, Nguyen HV, Van Horn CG, Hutson SM, Davis TA. Leucine and alpha-ketoisocaproic acid, but not norleucine, stimulate skeletal muscle protein synthesis in neonatal pigs. J Nutr. 2010;140:1418–1424. doi: 10.3945/jn.110.123042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flakoll P, Sharp R, Baier S, Levenhagen D, Carr C, Nissen S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition. 2004;20:445–451. doi: 10.1016/j.nut.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller J, Sharp R. Free acid gel form of beta-hydroxy-beta-methylbutyrate (HMB) improves HMB clearance from plasma in human subjects compared with the calcium HMB salt. Br J Nutr. 2011;105:367–372. doi: 10.1017/S0007114510003582. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586:6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell. 2012;149:410–424. doi: 10.1016/j.cell.2012.02.044. [DOI] [PubMed] [Google Scholar]
- Hao Y, Jackson JR, Wang Y, Edens N, Pereira SL, Alway SE. β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am J Physiol Regul Integr Comp Physiol. 2011;301:R701–R715. doi: 10.1152/ajpregu.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Van Hall G, Rose AJ, Miller BF, Doessing S, Richter EA, Kjaer M. Contraction intensity and feeding affect collagen and myofibrillar protein synthesis rates differently in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E257–E269. doi: 10.1152/ajpendo.00609.2009. [DOI] [PubMed] [Google Scholar]
- Hsieh L-C, Chien S-L, Huang M-S, Tseng H-F, Chang C-K. Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac J Clin Nutr. 2006;15:544–550. [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–31S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose–response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PE, Barber A, D’Olimpio JT, Hourihane A, Abumrad NN. Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine. Am J Surg. 2002;183:471–479. doi: 10.1016/s0002-9610(02)00823-1. [DOI] [PubMed] [Google Scholar]
- Murton AJ, Constantin D, Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim Biophys Acta. 2008;1782:730–743. doi: 10.1016/j.bbadis.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Nissen S, Sharp R, Ray M, Rathmacher JA, Rice D, Fuller JC, Connelly AS, Abumrad N. Effect of leucine metabolite beta-hydroxy-beta-methylbutyrate on muscle metabolism during resistance-exercise training. J Appl Physiol. 1996;81:2095–2104. doi: 10.1152/jappl.1996.81.5.2095. [DOI] [PubMed] [Google Scholar]
- Nissen S, Van Koevering M, Webb D. Analysis of beta-hydroxy-beta-methyl butyrate in plasma by gas chromatography and mass spectrometry. Anal Biochem. 1990;188:17–19. doi: 10.1016/0003-2697(90)90522-b. [DOI] [PubMed] [Google Scholar]
- Norton LE, Wilson GJ, Layman DK, Moulton CJ, Garlick PJ. Leucine content of dietary proteins is a determinant of postprandial skeletal muscle protein synthesis in adult rats. Nutr Metab (Lond) 2012;9:67. doi: 10.1186/1743-7075-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K, Barua JM, Watt PW, Scrimgeour CM, Rennie MJ. Flooding with L-[1-13C]leucine stimulates human muscle protein incorporation of continuously infused L-[1-13C]valine. Am J Physiol Endocrinol Meta. 1992;262:E372–E376. doi: 10.1152/ajpendo.1992.262.3.E372. [DOI] [PubMed] [Google Scholar]
- Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol Endocrinol Metab. 1998;275:E73–E88. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- Suryawan A, Jeyapalan AS, Orellana RA, Wilson FA, Nguyen HV, Davis TA. Leucine stimulates protein synthesis in skeletal muscle of neonatal pigs by enhancing mTORC1 activation. Am J Physiol Endocrinol Metab. 2008;295:E868–E875. doi: 10.1152/ajpendo.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Koevering M, Nissen S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am J Physiol Endocrinol Meta. 1992;262:E27–E31. doi: 10.1152/ajpendo.1992.262.1.E27. [DOI] [PubMed] [Google Scholar]
- Watt PW, Lindsay Y, Scrimgeour CM, Chien PA, Gibson JN, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: Use in studies of human tissue protein synthesis. Proc Natl Acad Sci U S A. 1991;88:5892–5896. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes EA, Selby AL, Atherton PJ, Patel R, Rankin D, Smith K, Rennie MJ. Blunting of insulin inhibition of proteolysis in legs of older subjects may contribute to age-related sarcopenia. Am J Clin Nutr. 2009;90:1343–1350. doi: 10.3945/ajcn.2009.27543. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jetton TL, Goshorn S, Lynch CJ, She P. Transamination is required for α-ketoisocaproate but not leucine to stimulate insulin secretion. J Biol Chem. 2010;285:33718–33726. doi: 10.1074/jbc.M110.136846. [DOI] [PMC free article] [PubMed] [Google Scholar]


