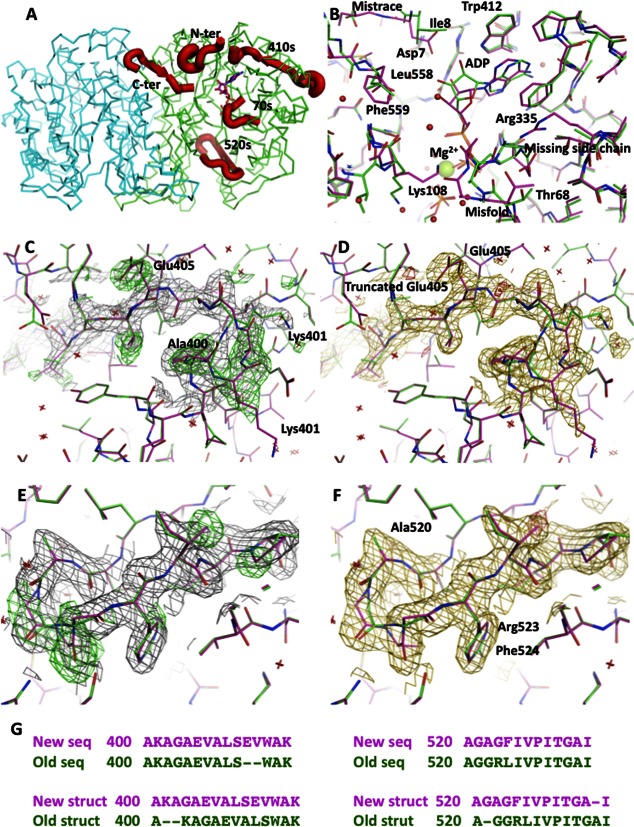
Figure 1.
(A) Model of the dimer of N10-formyltetrahydrofolate synthetase in carbon alpha representation with four misthreaded fragments marked in thick red ribbon. The stick representation of ADP marks the active site. (B) Superposition of the original and corrected structure with ADP at 2.5 Å resolution. The corrections lead to a significant repositioning of the ADP and a proper characterization of the metal ion as Mg2+. (C) The original model in green in the 400–410 region superimposed on the new model in purple, covered with the electron density calculated from the original model. The positive difference electron density contoured at 2.8 sigma level is in green. (D) The superimposed models at the same region as in (C) covered with the electron density calculated from the new model. The difference electron density contoured at 2.8 sigma level is in red. (E) The original model in green in the 520–530 region superimposed on the new model in purple, covered with the electron density calculated from the original model. The positive difference electron density contoured at 2.8 sigma level is in green. (F) The superimposed models at the same region as in (C) covered with the electron density calculated from the new model. The difference electron density contoured at 2.8 sigma level is in red. (G) The sequence corrections for 410 and 520 regions indicate the sequence variant for a clone ATCC 39073. Original sequence is in green and the new sequence is in purple, which corresponds to colors used in panels B–F. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]