Figure 1.
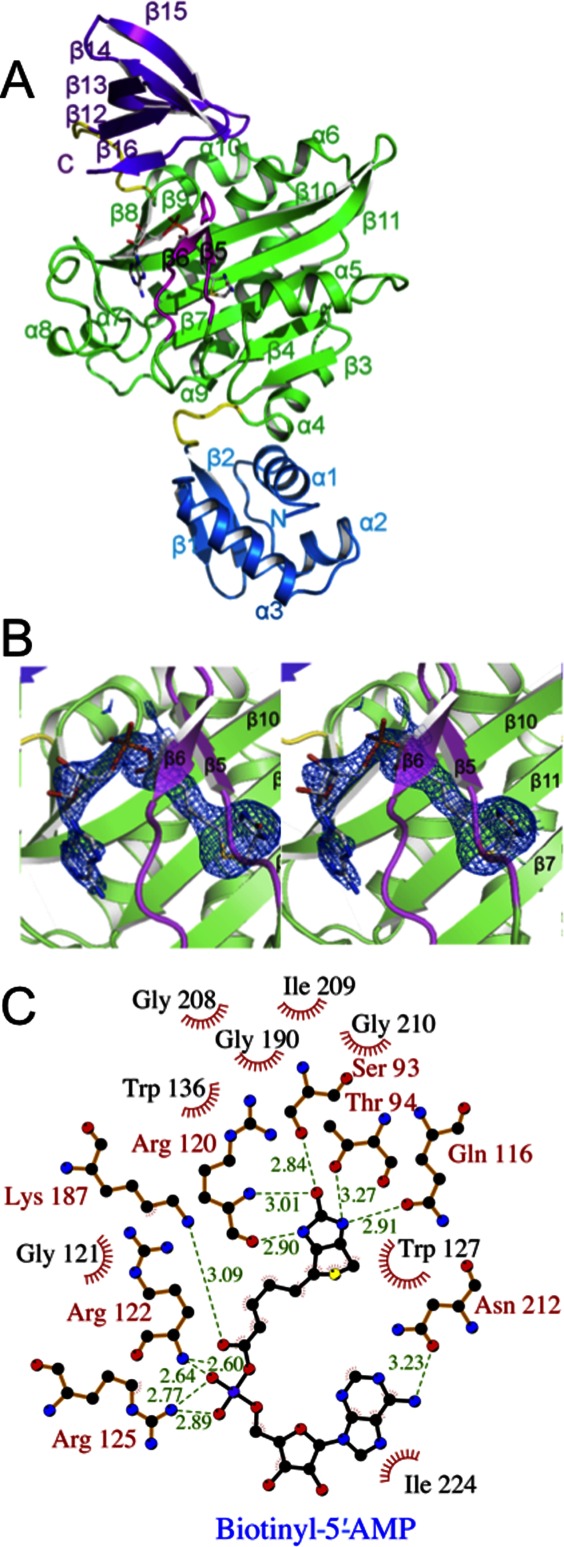
Crystal structure of SaBPL. (A) Cartoon schematic of Btyl-SaBPL monomer showing the N-terminal DNA-binding domain, residues 2–60 (blue), the central catalytic domain residues 75–268 (green), and the C-terminal cap domain 282–323 (purple). The random coil linkers residues 62–67 and 275–281 that connect the domains are in yellow. The corepressor biotinyl-5′-AMP is shown in stick representation. The BBL is highlighted in pink. (B) Cartoon schematic of the catalytic site of Btyl-SaBPL and the final 2Fo-Fc map (determined to 2.6 Å resolution and contoured at the 1 σ level) showing the positioning of biotinyl-5′-AMP (stick representation). (C) A schematic representation of contacts made to biotinyl-5′-AMP within the active site of SaBPL made using LIGPLOT.30 Residues involved in hydrogen bonding are annotated in red and hydrophobic interactions are annotated in black with red fray. Biotinyl-5′-AMP is shown with purple bonds and hydrogen bonding distance shown in Ångstroms by green dashed lines.
