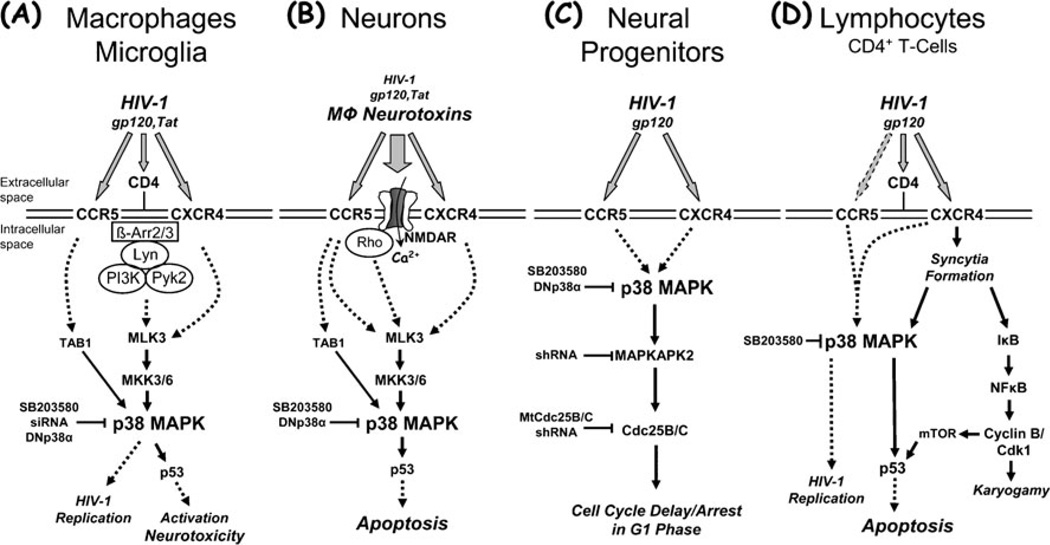
Fig. 1.
Summary of the apparent roles for p38 MAPK in HIV-1 infection and associated pathological processes potentially underlying brain injury, including macrophage activation and neurotoxicity, impairment of neurogenesis, and lymphocyte apoptosis. Activation of p38 MAPK by HIV-1 or its components can possibly contribute to the development of HAND and AIDS via various pathways. HIV-1 infection and replication in macrophages is associated with activation and production of neurotoxins (a). Macrophage neurotoxins induced by intact HIV-1 or its components, such as the envelope protein gp120 or the regulatory factor Tat, cause neuronal injury and death, and a direct effect of viral factors may contribute as well (b). HIV-1 or its coat protein gp120 inhibit proliferation of neural progenitors and thus compromise neurogenesis (c), and HIV-1 infection and replication in lymphocytes is associated with syncytia formation and apoptosis (d). HIV-infected and immune-activated, brain-infiltrating macrophages (MΦ) and microglia release presumably neurotoxic factors (reviewed in (Kaul et al. 2001; Gonzalez-Scarano and Martin-Garcia 2005; Kraft-Terry et al. 2009; Lindl et al. 2010)). The list of currently suspected neurotoxins includes quinolinic acid and other excitatory amino acids, such as glutamate and l-cysteine, arachidonic acid, platelet activating factor, a small molecule named ‘NTox’, free radicals, TNF-α, among others. These factors from MΦ/microglia (and also possibly from stimulated astrocytes) contribute to neuronal injury, dendritic and synaptic damage and loss, and apoptosis as well as possibly to astrocytosis. MΦ/microglia and lymphocytes express CCR5 and CXCR4 chemokine receptors on their surface in addition to CD4, and viral gp120 interacts via these receptors. Entry of HIV-1 into MΦ/microglia and lymphocytes occurs via gp120 binding, and therefore it is not surprising that gp120 (and Tat) are capable of activating uninfected MΦ/microglia to release similar factors to those secreted in response to productive HIV infection. In the same way, it may not be surprising that gp120 triggers apoptosis in infected and uninfected lymphocytes. Interestingly, although both CCR5-preferring and CXCR4-preferring HIV-1 variants infect lymphocytes depending on their expression profile of chemokine receptors, syncytia formation apparently occurs exclusively via CXCR4 and HIV/gp120 that preferentially interact with this receptor. Interestingly, Tat can apparently freely cross through cellular membranes by itself and thus potentially affect all cell types. However, Tat also interacts with chemokine receptors, including CXCR4, and NMDA receptors. Neurons (and astrocytes) also possess CXCR4 and CCR5 on their surface, raising the possibility of direct interaction with gp120 in the absence of CD4. Neuronal injury involves overactivation of NMDARs with resultant excessive influx of Ca2+ (reviewed in (Kaul et al. 2001, 2005; Kraft-Terry et al. 2009; Lindl et al. 2010)). This in turn leads to overactivation of a variety of potentially harmful signaling systems, among p38 MAPK. Interference of HIV-1/gp120 with the function of neural progenitor cells induces delay or even arrest of proliferation and promotes quiescence, but apparently does not result in apoptosis (Krathwohl and Kaiser 2004a, b; Okamoto et al. 2007). Solid arrows show direct interaction or effect, while dotted arrows indicate indirect or potential direct interaction. β-Arr β-Arrestin, Cdk1 cyclin-dependent kinase 1, DNp38α mutant, dominant-negative interfering p38α MAPK isoform, IκB inhibitor of NFκBMtCdc25B/C mutant Cdc25B/C, mTOR mammalian target of rapamycin, NFκB nuclear factor κB, shRNA small hairpin RNA, siRNA small interfering RNA, TAB1 transforming growth factor-β-activated protein kinase (TAK1) binding protein 1