Abstract
Previous imaging studies have suggested that there is an age-related decline in brain serotonin (5HT) measures in healthy subjects. This paper addresses whether 5HT1B receptor availability decreases with aging via positron emission tomography (PET) imaging.
Methods
48 healthy control subjects (mean 30±10 years; age range 18 to 61; 33 men, 15 women) completed [11C]P943 scans on a high resolution PET tomograph. Regions were examined with and without grey matter masking (GMM), the latter in an attempt to control for age related grey matter atrophy on binding potential (BPND) as determined by a validated multilinear reference tissue model (MRTM2).
Results
5-HT1B BPND receptor binding decreased in the cortex at an average rate of 8% per decade without and 9% with GMM. A negative association with age was also observed in all individual cortical regions. Differences in the putamen and pallidum (positive association) were significant following adjustment for multiple comparisons. No effects of 5-HT1B BPND were found with gender or race in any regions.
Conclusion
These findings indicate that age is a relevant factor for the 5-HT1B receptor in the cortex of healthy adults.
Keywords: Serotonin1B, 5-HT1B, PET imaging, [11C]P943, aging
Serotonin (5-Hydroxytryptamine or 5-HT) has been implicated in the aging of the brain. Preclinical and clinical work has shown alterations in functional and structural capacities of 5HT during aging (1,2). In translational neuroimaging, decreases in age have been found in the serotonin transporter, 5-HT1A and 5-HT2A receptors and cerebral glucose metabolism after administration of a SSRI in many cortical areas (3-8).
Recently, evidence of aging on the 5-HT system has also focused on the 5-HT1B receptor (1,9). 5-HT1B receptors are part of the (5-HT1) receptor family and are Gprotein coupled metabotropic receptors spanning seven transmembranes (10). 5-HT1B are expressed as auto-receptors on serotoninergic neurons where they are the predominant presynaptic modulator of 5-HT release in the brain and as heteroreceptors on nonserotoninergic neurons (10). While the only clinically available 5-HT1B medications available are for headaches (the triptan class of 5-HT1B/D agonists), these receptors have become increasingly important in neurophysiologic functions and for behaviors as diverse as locomotor activity, drug abuse reinforcement (11), depression (12) and PTSD (13) as well as learning, memory, and aggressive behavior (for a review, see 10).
Basic research in aging has shown large decreases (of up to 27%) in the density of 5-HT1B receptors (5HT1A did not apparently decrease) in elderly rats (9,14) and postsynaptic 5-HT1B mRNA in several brain regions (14), raising the possibility that aged animals may have relatively specific reduced 5-HT1B function. In addition, another study focused on 5HT1B and aging by employing 5HT1B KO mice to demonstrate that knockout mice had an early age-related motor decline and decreased life span, thus increasing the plausibility that 5HT1B is an important component of normal aging (1).
An interesting finding of the previous paper was a comparative phylogenetic conservation of a genetic age-effect from mice to humans suggesting that 5HT1B may be a modulator of aging across species (1). To date, however, possible effects of aging on 5HT1B have not been studied in healthy humans. The aim of this study was to investigate age effects in the brain of healthy subjects via the highly selective 5-HT1B ligand [11C]P943 using positron emission tomography (PET) imaging.
MATERIALS AND METHODS
SUBJECTS
Forty-eight healthy, medication-free volunteers were recruited through public advertisement to participate in the study. Subjects were between 18 and 61 years of age (mean± SD= 30 ± 10), 33 (69%) were males, and 39 (81%) were Caucasian, 5 (10%) were African-American, while 2 participants each were Hispanic, Asian-American, or multiracial. One subject (age 26) was excluded based on statistical grounds (> 3 standard deviations above mean cortical BPND values).
All subjects had a comprehensive screening assessment that included a psychiatric evaluation with a clinical interview and application of the SCID (Structured Clinical Interview for Diagnostic) for DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition) Axis I disorders, complete physical examination with medical history, routine blood tests, pregnancy test, urine toxicology and EKG. Individuals were excluded if they reported a diagnosis of current or lifetime Axis I or II psychiatric disorders; current or past serious medical or neurological illness (including a history of head injury with loss of consciousness); current pregnancy (as documented by pregnancy testing at screening and on the day of the PET imaging study) or breast feeding; general MRI exclusion criteria; and significant alcohol or illicit substance abuse or dependence in the past 3 months. All subjects were medication free for a minimum of 6 weeks at the time of the scan.
The study was performed under protocols approved by the Yale Human Investigation Committee, the Human Subjects Subcommittee of the Veterans Affairs Connecticut Healthcare System, the Yale University Radiation Safety Committee, the Yale-New Haven Hospital Radiation Safety Committee, and the Yale MRI safety committee. Subjects were recruited from New Haven and surrounding areas by advertisement, word of mouth and referrals. Written informed consent was obtained from all participants after a full explanation of study procedures.
RADIOCHEMISTRY
[11C]P943 (R-1-[4-(2-methoxy-isopropyl)-phenyl]-3-[2-(4-methyl-piperazin-1-yl)benzyl]-pyrrolidin-2-one) was prepared as previously described (15) by N-methylation of the precursor with [11C]methyl triflate, using the PETtrace cyclotron and a TRACERLab™ F×C automated synthesizer (GE Healthcare, Chalfont St. Giles, United Kingdom). The GE Microlab® was employed in some of the preparations as a source of the requisite [11C]methyl iodide.
SCANNING AND IMAGING PROCEDURES
PET imaging was performed with the selective 5-HT1B receptor antagonist radiotracer [11C]P943. All scans used a high-resolution research tomograph (HRRT) (Siemens/CTI, Knoxville, TN, USA), which acquired 207 slices (1.2mm slice separation) with a reconstructed image resolution of ~3mm. A transmission scan with a 137Cs point source was obtained before the emission scan. The PET scans were acquired for 120 min at rest with a single intravenous injection of 630 ± 132 MBq and a high specific activity of 161 ± 71 MBq/nmol.
Structural Magnetic resonance images were performed on a Siemens 3-T Trio system (Siemens Medical Solutions, Malvern, Pennsylvania) with a circularly polarized head coil for each subject to exclude individuals with anatomical abnormalities and for coregistration. The dimension and voxel size of MR images were 256 × 256 × 176 and 0.98 × 0.98 × 1.0 mm3, respectively.
Dynamic PET scan data were reconstructed with all corrections (attenuation; normalization; scatter; randoms; deadtime and motion), using the MOLAR algorithm (16) with the following frame timing: 6 × 30 sec; 3 × 1 min; 2 × 2 min; 22 × 5 min. Motion was additionally corrected by image smoothing (with a Gaussian filter at full width at half maximum (FWHM) of 3 mm and either used an optical detector (Vicra, NDI Systems, Waterloo, Ontario, Canada) or coregistered each frame image to an early summed image (0-10 min post-injection) using a 6-parameter mutual information algorithm (FLIRT, FSL 3.2, Analysis Group, FMRIB, Oxford, UK). Both motion corrections were added as a covariate in further analysis and no significant differences with found in any of the regions studied (F1,45=0.01, p=0.9144). As in previous P943 studies, PET data were used to produce a time–activity curve for the cerebellum, which was used as reference. The multilinear reference tissue model, MRTM2, has been previously validated in a P943 study and was used to produce parametric images of BPND (17).
A second summed image (0–10 min after injection) was created from the motion-corrected PET data and registered to the subject’s MR image, which in turn was registered (12-parameter affine transformation) to a MR template (Montreal Neurological Institute space).
Regions of interest were based on the Anatomical Automatic Labeling (AAL) template delineated on a MR template (18). The primary region was the cerebral cortex that was a summed result of the frontal, occipital, parietal, and temporal cortexes. A secondary analysis examined subcortical areas (amygdala, caudate, hippocampus, hypothalamus, pallidum, putamen, and thalamus). These results were obtained using individual parametric images that were resliced in template space using the PET to MR and the MR to template transforms.
In order to account for possible partial volume effects, a binary grey matter mask (GMM) was employed. For these results, individual MR images were segmented with FAST (FMRIB’s Automated Segmentation Tool, v3.1) to obtain gray, white, and CSF masks. The individual GMM images were then applied to the regions from the AAL template to obtain the mean regional values limited to grey matter voxels.
STATISTICAL ANALYSIS
All outcomes were summarized descriptively and assessed for normality prior to analysis using normal probability plots and Kolmogorov test statistics. All outcomes were approximately normal. Correlations between age and 5-HT1B were calculated separately within, and averaged across, each region. A linear mixed model was used to model the independent and joint effects of age (continuous) and cortical region (within-subject factor) on BPND values. The interaction between region and age was modeled and slopes for each cortical region were estimated post-hoc. Within-subject correlations were accounted for by fitting three variance-covariance structures to the data (unstructured, compound symmetry, and heterogeneous compound symmetry) and then selecting the best-fitting structure according to the Bayesian Information Criterion (BIC). Subcortical regions were analyzed as secondary subregions and adjusted using the Bonferroni correction. All analyses were conducted using SAS, version 9.1 (Cary, NC).
RESULTS
Table 1 presents average 5-HT1B (±S.D.) BPND levels for cortical and segmented cortical grey matter regions along with the slope and correlations with age. Significant negative associations between age and 5-HT1B BPND were observed in all cortical regions and total cortical BPND averaged an 8% decline per decade studied (9% in total cortical grey matter using GMM).
Table 1.
Regional mean BPND, values, correlations between age and [11C]P943 BPND values, and slope for cortical ROIs
|
Participants
(n=48) |
Mean BPND (S.D.) | Pearson R | Slope (S.E) | P Value |
|---|---|---|---|---|
| Cortical ROIs | ||||
| Frontal | .92 (.15) | −0.48 | −0.0067 (.002) | <0.0001 |
| Occipital | .85 (.13) | −0.53 | −0.0067 (.002) | <0.0001 |
| Parietal | .74 (.13) | −0.61 | −0.0076 (.002) | <.0.0001 |
| Temporal | .76 (.11) | −0.48 | −0.0053 (.002) | <0.001 |
| Total Cortex | .82 (.15) | −0.52 | −0.0066 (.001) | <0.0001 |
| Cortical ROIs (grey matter masked) |
||||
| Frontal | 1.25 (.15) | −0.62 | −0.0087 (.002) | <0.0001 |
| Occipital | 1.03 (.14) | −0.56 | −0.0075 (.002) | <0.0001 |
| Parietal | .92 (.13) | −0.66 | −0.0084 (.001) | <0.0001 |
| Temporal | .88 (.11) | −0.52 | −0.0052 (.001) | <0.0001 |
| Total Cortex | .99 (.16) | −0.59 | −0.0075 (.001) | <0.0001 |
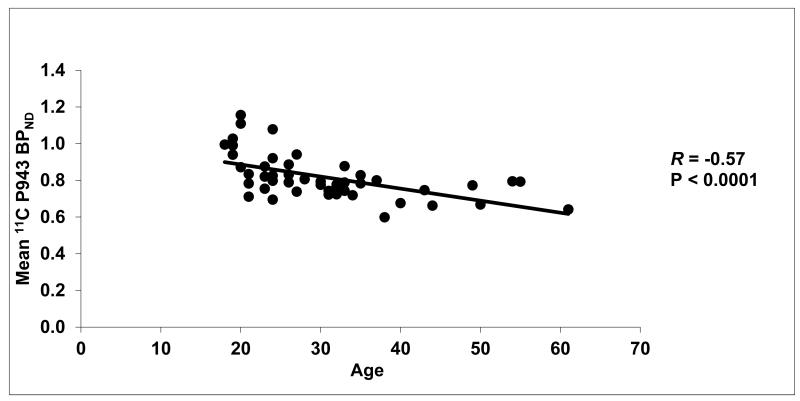
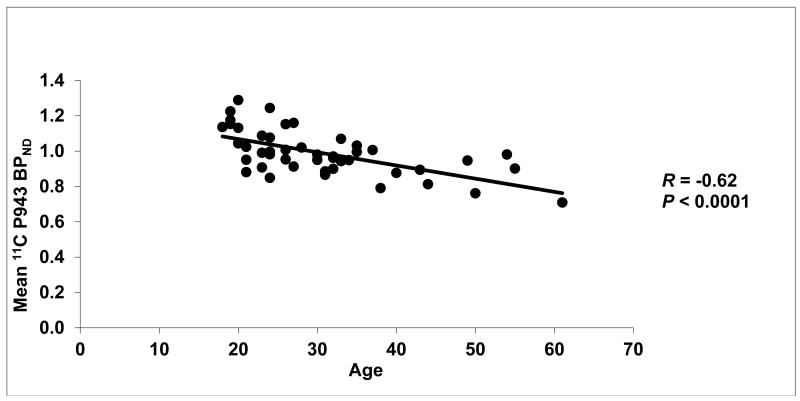
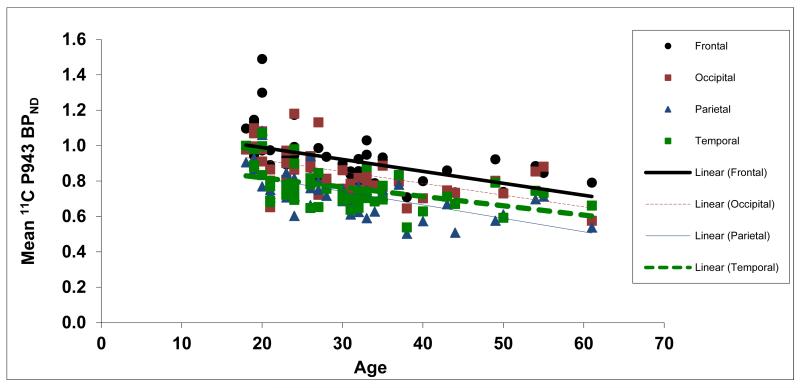
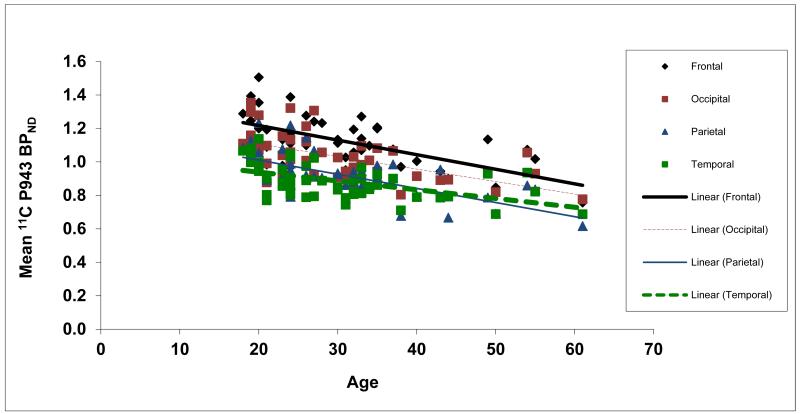
Results of the primary mixed model showed an overall significant effect of age on both total BPND (F1,45=21.6, p<0.0001) and total BPND after GMM (F1,45=28.6, p<0.0001), averaged across all cortical regions. These effects are depicted in Figures 1 and 2, respectively. Consistent with the univariate results, primary mixed model slope estimates for the individual 5-HT1B BPND cortical regions (with and without GMM) remained significant (p=<0.01) with age (Figures 3 and 4, respectively).
Figure 1.
Mean cortical BPND values for each subject along with their age (in years).
Figure 2.
Mean cortical BPND values (after a grey matter mask) for each subject along with their age (in years).
Figure 3.
Mean BPND values for each cortical region along with age (in years).
Figure 4.
Mean BPND values (after a grey matter mask) for each cortical region along with age (in years).
Subcortical region (without GMM) correlations were largely insignificant when age and 5-HT1B BPND values were examined with the exception of the hypothalamus (r=−0.28, p=0.05), pallidum (r=+0.39, p=0.01) and putamen (r=+0.41, p<0.01). GMM subcortical results found a difference in the putamen (r=+0.32, p=0.03) and a trend level difference in the pallidum (r=+0.26, p=0.08) with all other regions nonsignificant. Subcortical results remained significant after a multiple comparison correction in the pallidum (Bonferroni adjusted p=0.05) and putamen (Bonferroni adjusted p=0.03) without GMM.
Gender or race had no significant effects on the above results.
DISCUSSION
This work has furthered the role of aging on the serotonin system by demonstrating age effects on 5HT1B with the PET radiotracer [11C]P943. This change in BPND was found throughout cortical areas with increased age. These findings are consistent with the existing literature on aging and serotonin that have shown decreases in the density of 5HT1A and 5HT2A receptors in aging (3-6, 8, 19).
Different methods were employed in the above studies to control for cerebral atrophy. The current work used a grey matter segmentation process to create a grey matter binary mask and while this method is not necessarily sensitive to all partial volume effects, the retention of findings argues against cerebral atrophy being responsible for the decreases in 5HT1B with aging. Furthermore, a recent study that examined 5HT2A and aging found that a partial volume correction was not necessary when similar parameters (cortical ROIs, high resolution scanning, and a healthy population) were examined (19).
Our sample was composed of mostly male subjects (69%) that were younger than 61 years and was also limited in distribution across age regions (with the majority of subjects under 40). This is a limitation to the current analysis as it is susceptible to bias induced by fewer older subjects and gender (although no current gender effects were found). Further studies, including a more balanced distribution of older subjects, could be justified to verify that 5HT1B receptor decline is a linear phenomenon in advanced age. While the MRTM2 reference model is a validated method to examine this data, another potential limitation in this study was the lack of available arterial blood flow data in the majority of subjects to rule out potential confounds such as age-related effects of the cerebellar time activity curve.
The preliminary finding of increased 5HT1B BPnd in the pallidum and putamen (which retained significance after multiple corrections) could be of interest as there are no current reports of increasing serotonin receptor brain levels with age in any region. If confirmed in further work, these subcortical areas could be a focus of a potentially novel process.
Finally, while the focus of this paper was to investigate age effects on 5-HT1B receptors, the functional impact of this finding could extend into cognitive effects such as memory or learning which have been implicated with 5-HT1B decline in a host of preclinical work (1,9,14). In the only other work to date on an aged human population, Alzheimer’s patients were found postmortem to have reduced 5-HT1B receptor density compared to controls in the frontal and temporal cortexes (20). Interesting, cognitive decline was associated with more 5-HT1B receptors in this study. The authors attributed this finding to a compensation of 5-HT1B hetroreceptors to a deteriorated cholinergic system (i.e. that the hetroreceptors, which function to inhibit acetylcholine, led to worse cognitive performance due to decreased acetylcholine in an already cholinergic deficit state in Alzheimer’s.) It remains to be seen if 5-HT1B medications could have a clinical effect for age specific cognitive problems, but the current results support further investigation of this potential learning and memory treatment.
ACKNOWLEDGEMENTS
Both the P943 standard and the N-desmethyl-precursor were provided by Pfizer, Inc (Groton, CT, USA).
Disclosures: The project was supported by the National Institutes of Health through the following awards: R21 MH081103 (ARRA), R21 MH085627, and R21 AA018329. Zubin Bhagwagar is a full time employee at Bristol-Meyers Squibb. Espen Walderhaug is supported through the Research Council of Norway, Division for Science.
REFERENCES
- 1.Sibille E, Su J, Leman S, et al. Lack of serotonin1B receptor expression leads to age-related motor dysfunction, early onset of brain molecular aging and reduced longevity. Mol Psychiatry. 2007;11:1042–1056. doi: 10.1038/sj.mp.4001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meltzer CC, Reynolds CF., 3rd. In vivo assessment of aging changes in serotonin function. Neuropsychopharmacology. 1999;21:323–324. doi: 10.1016/S0893-133X(99)00058-5. [DOI] [PubMed] [Google Scholar]
- 3.Meltzer CC, Smith G, Price JC, Reynolds CF, III, Mathis CA, Greer P, et al. Reduced binding of [18F]altanserin to serotonin type 2A receptors in aging: persistence of effect after partial volume correction. Brain Res. 1998;813:167–171. doi: 10.1016/s0006-8993(98)00909-3. [DOI] [PubMed] [Google Scholar]
- 4.Versijpt J, Van Laere KJ, Dumontc F, et al. Imaging of the 5-HT2A system: age-, gender-, and Alzheimer’s disease-related findings. Neurobiol Aging. 2003;24:553–561. doi: 10.1016/s0197-4580(02)00137-9. [DOI] [PubMed] [Google Scholar]
- 5.Tauscher J, Verhoeff NP, Christensen BK, Hussey D, Meyer JH, Kecojevic A, et al. Serotonin 5-HT1A receptor binding potential declines with age as measured by [11C]WAY-100635 and PET. Neuropsychopharmacology. 2001;24:522–530. doi: 10.1016/S0893-133X(00)00227-X. [DOI] [PubMed] [Google Scholar]
- 6.Baeken C, D’haenen H, Flamen P, Mertens J, Terriere D, Chavatte K, et al. 123I-5-IR91150, a new single-photon emission tomography ligand for 5-HT2A receptors: influence of age and gender in healthy subjects. Eur J Nucl Med. 1998;25:1617–1622. doi: 10.1007/s002590050339. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg S, Smith GS, Barnes A, et al. Serotonin modulation of cerebral glucose metabolism in normal aging. Neurobiol Aging. 2004;25:167–174. doi: 10.1016/s0197-4580(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 8.Costes N, Merlet I, Ostrowsky K, et al. A 18F MPPF PET normative database of 5-HT1A receptor binding in men and women over aging. J Nucl Med. 2005;46:1980–1989. [PubMed] [Google Scholar]
- 9.David DJ, Bourin M, Hascoet M, et al. Comparison of antidepressant activity in 4- and 40-week-old male mice in the forced swimming test: involvement of 5-HT1A and 5-HT1B receptors in old mice. Psychopharmacology (Berlin) 2001;153:443–449. doi: 10.1007/s002130000588. [DOI] [PubMed] [Google Scholar]
- 10.Sari Y. Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev. 2004;28:565–582. doi: 10.1016/j.neubiorev.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Henry S, Gallezot J-D, et al. Serotonin 1B receptor imaging in alcohol dependence. Biol Psychiatry. 2010;67:800–803. doi: 10.1016/j.biopsych.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murrough JW, Henry S, Hu J, et al. Reduced ventral striatal/ventral pallidal serotonin1B receptor binding potential in major depressive disorder. Psychopharmacology. 2011;213:547–553. doi: 10.1007/s00213-010-1881-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murrough JW, Czermak C, Henry S, et al. Age at first trauma is associate with serotonin 1B receptor reductions and comorbid depression in posttraumatic stress disorder. Arch Gen Psychiatry. 2011;68:892–900. doi: 10.1001/archgenpsychiatry.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitchell ES, McDevitt RA, Neumaier JF. Adaptations in 5-HT receptor expression and function: Implications for treatment of cognitive impairment in aging. Neurosci Research. 2009;87:2803–2811. doi: 10.1002/jnr.22100. [DOI] [PubMed] [Google Scholar]
- 15.Nabulsi N, Huang Y, Weinzimmer D, et al. High resolution imaging of brain 5-HT1B receptors in the rhesus monkey using [11C]P943. Nucl Med Biol. 2010;37:205–214. doi: 10.1016/j.nucmedbio.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson CA, Thada S, Rodriguez, et al. Software architecture of the MOLAR-HRRT reconstruction engine. IEEE Nucl. Sci. Symp. Conf. Rec. 2004;6:3956–3960. [Google Scholar]
- 17.Gallezot JD, Nabulsi N, Neumeister A, et al. Kinetic modeling of the serotonin 5-HT(1B) receptor radioligand [(11)C]P943 in humans. J Cereb Blood Flow Metab. 2010;30:196–210. doi: 10.1038/jcbfm.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 19.Uchida H, Chow TW, Mamo DC, et al. Effects of aging on 5-HT2AR binding: a HRRT PET study with and without partial volume corrections. Int J Geriatr Psychiatry. 2011;26:1300–1308. doi: 10.1002/gps.2682. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Alloza M, Hirst WD, Chen CPLH, et al. Differential involvement of 5-HT1B/1D and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer’s disease. Neuropsychopharmacology. 2004;29:410–416. doi: 10.1038/sj.npp.1300330. [DOI] [PubMed] [Google Scholar]