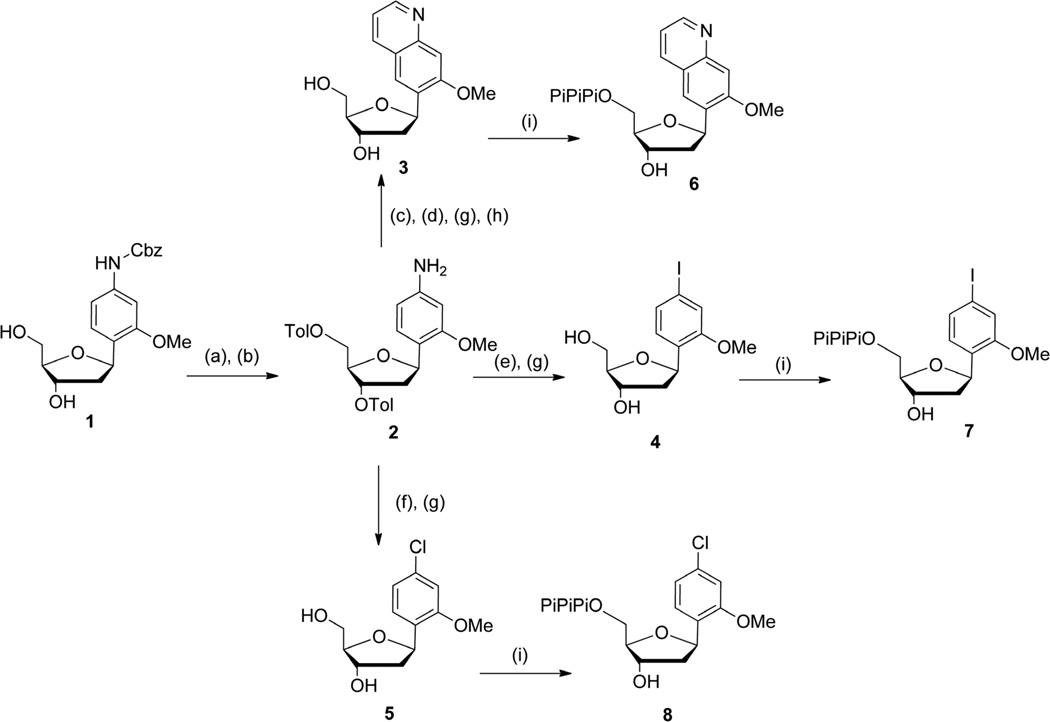
Scheme 1.
Conditions: (a) Toluyl chloride, pyridine, rt, 15 h, 59%; (b) 10% Pd/C, H2, EtOAc, NEt3, rt, 1 h, 91%; (c) (1) TsCl, pyr, CH2Cl2, rt, 40 min; (2) acrolein, NEt3, MeOH, 0 °C → rt, 20 min, 85%; (d) HCl 3N, THF, 80 °C, 40 min, 80%; (e) HCl aq 6M, NaNO2, KI, THF, 0 °C → rt, 2h, 55%; (f) HCl aq 6M, NaNO2, CuCl, THF, 0 °C → 40 °C, 5h, 23%; (g) MeONa 30% in MeOH, MeOH:CH2Cl2 8:2, 5 °C → rt, 30 min − 1 h: 3, 90%; 4, 86%; 5, 96%; (h) tBuOK, THF, 70 °C, 3 h, 78%; (i) proton sponge, POCl3, PO(OMe)3, −15 °C ° −10 °C, 3 h then Bu3N, (Bu3NH)2H2P2O7 in DMF, −10 °C → 0 °C, 30 min then TEAB buffer (0.5M), rt, 10 min: 6, 27%; 7, 48%; 8, 57%.