Abstract
Background and Aims
A positive correlation between tissue thickness and crassulacean acid metabolism (CAM) expression has been frequently suggested. Therefore, this study addressed the question of whether water availability modulates photosynthetic plasticity in different organs of two epiphytic orchids with distinct leaf thickness.
Methods
Tissue morphology and photosynthetic mode (C3 and/or CAM) were examined in leaves, pseudobulbs and roots of a thick-leaved (Cattleya walkeriana) and a thin-leaved (Oncidium ‘Aloha’) epiphytic orchid. Morphological features were studied comparing the drought-induced physiological responses observed in each organ after 30 d of either drought or well-watered treatments.
Key Results
Cattleya walkeriana, which is considered a constitutive CAM orchid, displayed a clear drought-induced up-regulation of CAM in its thick leaves but not in its non-leaf organs (pseudobulbs and roots). The set of morphological traits of Cattleya leaves suggested the drought-inducible CAM up-regulation as a possible mechanism of increasing water-use efficiency and carbon economy. Conversely, although belonging to an orchid genus classically considered as performing C3 photosynthesis, Oncidium ‘Aloha’ under drought seemed to express facultative CAM in its roots and pseudobulbs but not in its leaves, indicating that such photosynthetic responses might compensate for the lack of capacity to perform CAM in its thin leaves. Morphological features of Oncidium leaves also indicated lower efficiency in preventing water and CO2 losses, while aerenchyma ducts connecting pseudobulbs and leaves suggested a compartmentalized mechanism of nighttime carboxylation via phosphoenolpyruvate carboxylase (PEPC) (pseudobulbs) and daytime carboxylation via Rubisco (leaves) in drought-exposed Oncidium plants.
Conclusions
Water availability modulated CAM expression in an organ-compartmented manner in both orchids studied. As distinct regions of the same orchid could perform different photosynthetic pathways and variable degrees of CAM expression depending on the water availability, more attention should be addressed to this in future studies concerning the abundance of CAM plants.
Keywords: Cattleya walkeriana, crassulacean acid metabolism, drought, epiphytic orchid, leaf succulence, non-leaf photosynthesis, Oncidium ‘Aloha’, photosynthetic plasticity
INTRODUCTION
Epiphytic orchids are one of the most species-rich and diverse groups of plants and can inhabit a wide range of niches, varying from almost constantly humid to seasonally dry habitats. Despite the abundance of orchid species occupying the canopy, the epiphytic habitat is the most severe niche in tropical forests because the availability of water and nutrients is sporadic and dependent on atmospheric sources (Kress, 1986; Goh and Kluge, 1989; Benzing, 1990; Gravendeel et al., 2004). Hence, survival and adaptive success of these plants rely largely on their flexible developmental and metabolic responses to environmental conditions (Goh and Kluge, 1989; Benzing, 1990; Sinclair, 1990).
In terms of metabolic strategies, a high number of epiphytic species perform crassulacean acid metabolism (CAM) photosynthesis (Benzing, 1989; Zotz and Hietz, 2001; Lüttge, 2004), which represents an important ecophysiological adaptation that allows plants to reside in habitats with scarce, intermittent and/or seasonal water availability (Cushman, 2001; Silvera et al., 2010a; Borland et al., 2011). In fact, the drought endurance observed in the majority of epiphytes is frequently provided by a stronger CAM photosynthetic behaviour that promotes maximum carbon gain combined with minimum water loss (Benzing and Ott, 1981; Dodd et al., 2002; Kerbauy et al., 2012). This is feasible because CAM photosynthesis usually acts as a CO2-concentrating mechanism through nocturnal CO2 fixation by phosphoenolpyruvate carboxylase (PEPC) and subsequent vacuolar storage of the fixed CO2 in the form of organic acids. The following daytime decarboxylation of organic acids releases CO2, which is refixed by ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and assimilated in the Calvin cycle behind closed stomata (Griffiths, 1989; Lüttge, 2002, 2004).
Concurrently, one essential structural adaptation of most epiphytic orchids against severe drought is a certain degree of tissue succulence, mainly by their leaves and pseudobulbs (Goh and Kluge, 1989; Benzing, 1990). Besides being an efficient way to store water and nutrients during the dry season, succulence is also suggested as an important requirement for CAM expression (Dressler, 1981, 1993; Williams et al., 2001; Griffiths et al., 2008; Silvera et al., 2010a; Borland et al., 2011). In fact, a number of reports have indicated a positive correlation between leaf thickness and CAM activity in epiphytic orchids. In addition, it is currently accepted that orchid leaves can show either CAM or C3 photosynthesis depending on the presence of thick and thin leaves, respectively (Neales and Hew, 1975; Arditti, 1979; Goh et al., 1983). Furthermore, the higher leaf thickness of plants performing CAM is also related to an increased capacity to nocturnally store organic acids inside the vacuoles during the CAM cycle (Nelson et al., 2005; Nelson and Sage, 2008; Silvera et al., 2010b). However, this correlation seems to be exclusively valid when leaf succulence is due to increases in chlorenchyma thickness rather than hydrenchyma abundance (Kerbauy et al., 2012).
CO2 fixation in orchids has been primarily studied in leaves; relatively little attention has been given to the photosynthetic pathways occurring in non-leaf organs, such as pseudobulbs or roots. Although pseudobulbs lack stomata and are impervious to water and gases (Withner, 1974; Hew and Yong, 1994; Ng and Hew, 2000), anatomical and biochemical analyses have revealed the presence of chloroplasts and enzyme activities (Rubisco and PEPC), which, taken together, indicates some photosynthetic activity in these organs (Winter et al., 1983; Stern and Morris, 1992; Hew and Yong, 1994; Sheehan and Sheehan, 1994; Hew et al., 1996). This implies that, in some cases, pseudobulbs might also be capable of expressing some degree of CAM. Moreover, green aerial roots of epiphytic orchids also have the photosynthetic apparatus for CO2 fixation (Ho et al., 1983; Moreira et al., 2009; Martin et al., 2010) as well as several morphological specializations, such as velamen and exodermis, both designed to trap and absorb water and nutrients (Pridgeon, 1986). However, aerial roots seem to play a minor photosynthetic role for epiphytes (Aschan and Pfanz, 2003), except for some leafless orchids with autotrophic roots. These plants are almost totally dependent on carbon fixation by roots, which can exhibit CAM photosynthesis, even being devoid of true stomata (Benzing et al., 1983; Cockburn et al., 1985). Additionally, roots of some leafless orchids possess well-developed aeration systems with specialized thickened cortical cells that possibly are able to regulate gas exchange (Benzing et al., 1983).
Phylogenetic studies concerning the evolution of photosynthetic pathways in Orchidaceae leaves have shown C3 as the ancestral state and CAM as having multiple-independent origins with several reversals across the entire family, indicating the great evolutionary flexibility of CAM among orchids. Accordingly, the Epidendroideae subfamily of Orchidaceae is the most species-rich epiphytic clade among all plant groups, which correlates with expressive events of CAM radiation, especially within the Neotropical subtribes Oncidiinae and Laeliinae (Silvera et al., 2009). Moreover, approx. 40 % of the tropical orchid species are considered capable of expressing some form of CAM (‘strong’ to ‘weak’) in their leaves (Silvera et al., 2005, 2010a).
It is also important to note that several studies have shown that the extent of CAM expression can be highly flexible and dependent not only on plant species but also on tissue characteristics, the phase of organ/plant development and environmental conditions (Nelson and Sage, 2008; Winter et al., 2008; Herrera, 2009; Freschi et al., 2010a, b; Ping et al., 2010; Borland et al., 2011). Although some valuable investigations have already been performed to verify the expression of CAM in orchid tissues (Goh et al., 1983; Ando and Ogawa, 1987; Cui et al., 2004; Guo and Lee, 2006; Motomura et al., 2008; Moreira et al., 2009; Silvera et al., 2009; Martin et al., 2010), few studies have verified C3 and/or CAM photosynthetic types in different organs of the same epiphytic orchid or considered that these organs might have plasticity in switching between C3 and CAM pathways in response to changes in the environmental conditions (Kerbauy et al., 2012), such as drought.
Moreover, CAM plants show evolutionary convergence in terms of particular anatomical and metabolic traits and some of these traits (i.e. succulence and intercellular air space in photosynthesizing tissues) are recognized as putative determinants to constrain the range of photosynthetic plasticity performed by specific plant tissues (Nelson et al., 2005; Nelson and Sage, 2008; Borland et al., 2011). In fact, we have previously reported the existence of distinct degrees of CAM expression in different portions along the leaf blade of the epiphytic bromeliad Guzmania monostachia that were modulated by water availability. The drought treatment intensified the CAM expression in this C3–CAM facultative species, specifically in the apical part of leaves which had a set of physiological and morphological features considered more suitable for the occurrence of CAM photosynthesis (Freschi et al., 2010b). This information prompted us to further investigate whether other epiphytes with highly diverse and specialized physiology and morphology, as found among orchids (Kress, 1986; Gravendeel et al., 2004), would also respond to distinct regimes of water availability by exhibiting photosynthetic plasticity and functional compartmentalization of CO2 fixation in their vegetative tissues.
Based on previous information, the current study investigated the degree to which the photosynthetic modes (C3 and/or CAM) observed in vegetative organs of two epiphytic orchids from the subfamily Epidendroideae (Cattleya walkeriana, subtribe Laeliinae; Oncidium ‘Aloha’, subtribe Oncidiinae) with different degrees of leaf succulence are modulated by water availability. The seasonally dried epiphytic orchid C. walkeriana, as with other Cattleya species, is characterized by a high degree of leaf succulence and by performing constitutive CAM photosynthesis (Nuerenbergk, 1963; Arditti, 1979; Avadhani and Arditti, 1981; Avadhani et al., 1982; Goh and Kluge, 1989), while most Oncidium orchids have thinner leaves and are generally described as C3 plants (Hew and Yong, 1994, 2004). In fact, the thin-leaved hybrid Oncidium ‘Aloha’ is derived from the varieties Oncidium ‘Star Wars’ and Oncidium ‘Goldiana’ (Wu et al., 2010), the last being largely described as a C3-shade epiphytic orchid (Hew and Yong, 1994; Yong and Hew, 1995a, b). Meanwhile, the Brazilian species C. walkeriana is usually found in more exposed canopy sites of tropical deciduous forests (Lacerda, 1995). However, both orchids present large heteroblastic pseudobulbs and green roots (Goh and Kluge, 1989; Hew and Yong, 2004), thereby implying some potential for non-leaf photosynthetic activity.
Accordingly, we set out to discover (1) whether distinct regimes of water availability influence the photosynthetic mode (C3 and/or CAM) in leaves and non-leaf organs of both C. walkeriana and Oncidium ‘Aloha’, if so, (2) how drought specifically affects the photosynthetic pathway in leaves of a thick-leaved (Cattleya) and a thin-leaved (Oncidium) epiphytic orchid, and (3) whether there is a particular degree of compartmentalization in CAM expression among different organs of such epiphytic orchids under either drought or well-watered treatments. Therefore, the present study aimed to study whether a correlation exists between tissue organization and photosynthetic operation (C3 and/or CAM) in different vegetative organs (leaves, pseudobulbs and roots) of the epiphytic orchids C. walkeriana (thick leaved) and Oncidium ‘Aloha’ (thin leaved) subjected to both well-watered and drought conditions.
MATERIALS AND METHODS
Plant material and growth conditions
Plants of Cattleya walkeriana and Oncidium ‘Aloha’ (O. ‘Star Wars’ × O. ‘Goldiana’) were obtained by asymbiotic germination and micropropagation techniques, respectively. The plants were cultivated in pots containing moss substrate in greenhouses at São Paulo State until they reached the mature-vegetative age. These orchids were transferred to controlled environment chambers at 25 ± 2 °C with 12-h photoperiod and photosynthetic flux density of about 200 µmol photons m−2 s−1 supplied by fluorescent lamps (Sylvania, Germany). The plants were watered daily and acclimatized under such conditions for 15 d prior to the treatments. All orchids used in this study were in the rest period of their developmental cycle.
Morphological and histological analyses
The youngest, completely expanded leaf of individuals of C. walkeriana and Oncidium ‘Aloha’ and their respective pseudobulb and light-exposed roots were fixed in FAA (formalin, acetic acid and ethanol 50 %) for 24 h (Johansen, 1940) and BNF (buffered neutral formalin) for 48 h (Lillie, 1965). After fixation, leaf samples were dehydrated through a tertiary butyl alcohol series (Johansen, 1940), embedded in paraffin and serial sectioned at 20 µm thickness on a Microm HM340E rotary microtome. Longitudinal and transverse sections were stained with Astra blue 1 % and Safranin O 1 % (Gerlach, 1984) and the slides mounted in Permount. Some material was also freehand sectioned to carry out the histochemical test with Alcian blue (Pearse, 1985) for acidic mucilage and phloroglucinol (Johansen, 1940) for lignin. Observations and photographs were made with an Olympus BX51 light microscope. For scanning electron microscopy (SEM) analysis, samples fixed in FAA were dehydrated in a graded ethanol series and critical-point dried with CO2, attached to aluminium stubs and coated with gold (30–40 nm). Observations were carried out on a Jeol JSM-5800 LV.
Treatments and tissue sampling for biochemical analyses
Both sets of epiphytic orchids were divided into two experimental groups, each one with five plants submitted to a different watering condition. The well-watered plants were watered daily and maintained under 60–70 % relative humidity, while the drought-treated plants were submitted to 30–40 % relative humidity without watering. After 30 d under these treatments, the youngest leaf and pseudobulb and all light-exposed roots of these orchids were harvested at both dark-to-light and light-to-dark transitions. Roots immersed in the substrate were not used. All harvested samples were immediately fragmented into small pieces of about 2–5 mm and subsequently weighed, frozen in liquid nitrogen and stored at –80 °C until used in the biochemical analyses.
Tissue water content measurements
The tissue water content of the leaves, pseudobulbs and roots was determined according to Barrs and Weatherley (1962). Discs of approx. 1·53 cm2 of leaves and pseudobulbs and roots of 1 cm length from five different plants were collected 1 h after dawn and immediately weighed to determine the fresh mass (Mf). After determining Mf, the samples were maintained for 24 h in distilled water and in the dark for saturation to obtain the turgid mass (Mt) and then dried to a constant mass at 60 °C and allowed to cool before determining the dry mass (Md). Tissue water content was calculated using the formula [(Mf – Md)/(Mt – Md)] × 100.
PEPC and MDH extraction and assay
Quantification of both PEPC (EC 4.1.1.31) and malate dehydrogenase (MDH, EC 1.1.1.37) activities was performed according to the method described by Freschi et al. (2010a) with some modifications. For this, 1 g of fresh leaf or pseudobulb or 0·25 g of fresh root was ground in a mortar with liquid nitrogen and polyvinylpolypyrrolidone (PVPP) (0·3 g per fresh mass) until a fine powder was obtained. Frozen samples of leaves, pseudobulbs and roots collected at the dark-to-light transition were extracted in five volumes (v/w) of buffer containing 200 mm Tris-HCl (pH 8·0), 1 mm EDTA, 5 mm dithiothreitol (DTT), 10 mm MgCl2, 10 % (v/v) glycerol and 0·5 % (w/v) bovine serum albumin. The homogenate was filtered using Millex GV 0·45-μm filters (Millipore), and the supernatant was immediately used for the enzymatic assays. PEPC activity was assayed in a 2-mL standard reaction medium containing 50 mm Tris-HCl (pH 8·0), 1 mm DTT, 10 mm MgCl2, 10 mm NaHCO3, 200 mm NADH, 3 mm phosphoenolpyruvate and 0·005 units l-MDH (Sigma-Aldrich). MDH activity was assayed in the oxaloacetate (OAA)-reducing direction in a 2-mL reaction medium containing 50 mm Tris-HCl (pH 8·0), 2 mm OAA, 5 mm MgCl2 and 200 mm NADH. For both enzymatic determinations, the reaction was started by adding an aliquot of enzyme extract, and absorbance was continuously measured at 340 nm. All reported rates were from linear portions of absorbance time curves (usually between 0 and 5 min). The PEPC and MDH enzymes were assayed at 30 °C and their activities were expressed in μmol NADH min−1 (mg total chlorophyll)−1.
Organic acid quantification
Organic acids were quantified according to the method used by Amorós et al. (2003) and Hasegawa et al. (2010). For this, leaf (1 g), pseudobulb (1 g) or root (0·25 g) samples were ground in a mortar with liquid nitrogen and 0·06 g PVPP per gram of fresh mass. When a fine powder was obtained, the samples were transferred to previously cooled micro-tubes, and 600 µL of 0·662 mm formic acid solution was added to each tube. The extracts were mixed vigorously, and an aliquot of cold formic acid solution was added (400 µL). All tubes were centrifuged at 14 000 g for 6 min at 4 °C. The supernatant was collected and mixed with 200 µL AG3-X4 resin (Bio-Rad) (1 g resin per 4 mL cold distilled water) to extract the acid organics from the mucilage present in large quantities in the orchid tissues. The samples were kept under constant agitation at 4 °C. After 1 h, all tubes were centrifuged at 14 000 g for 4 min at 4 °C. The supernatant was discarded, and the pellet was re-suspended in 200 µL of the mobile phase (1 % H3PO4 solution, filtered in LCR PTFE 0·45-μm filters; Millipore). The tubes were maintained under constant agitation at 4 °C for 1 h, and the samples were then centrifuged at 14 000 g for 4 min at 4 °C. The supernatant was collected and filtered using Millex GV 0·22- or 0·45-μm filters (Millipore) to remove the resin particles from the extract. Aliquots of 50 µL were injected into a high-performance liquid chromatograph (Waters HPLC, 510 pump, UV/Vis 486 detector, 717 plus autosampler, and Millenium 32 software) equipped with an Aminex HPX-87H ion exclusion column (300 × 7·8 mm; Bio-Rad). The run was carried out isocratically at a flow rate of 0·5 mL min−1 and analysed in a UV/Vis detector settled to 215 nm. The column compartment temperature was adjusted to 28 °C. Corrections were made using formic acid as an internal standard.
Leaf gas exchange measurements
As previous photosynthetic experiments with Oncidium ‘Goldiana’ have shown that the leaf tip had the highest photosynthetic capacity along the leaf blade (Hew et al., 1998), gas exchange measurements were made on the apical portion of the youngest, completely expanded leaf of Oncidium ‘Aloha’ plants. This analysis was performed continuously using a portable infra-red gas exchange system (LI-6400, Li-Cor) with all parameters settled as described by Freschi et al. (2010b). Every analysed leaf was enclosed in a chamber (leaf area within the cuvette was always 4·5 cm2), which tracked the environmental conditions inside the growth cabinet. Leaf gas exchange parameters were logged automatically every 3 min, and a CO2 cylinder was used to keep CO2 concentration constant under 380 p.p.m. Measurement intervals were integrated to show hourly averages.
Statistical analyses
Data are presented as the mean ± s.e. of three replicate samples, with each replicate consisting of plant material collected from five different individuals. Statistically significant differences between means of well-watered and drought-exposed treatments were determined by Student's t-test at P ≤ 0·05.
RESULTS
Morpho-histological characteristics of orchid organs
Leaves
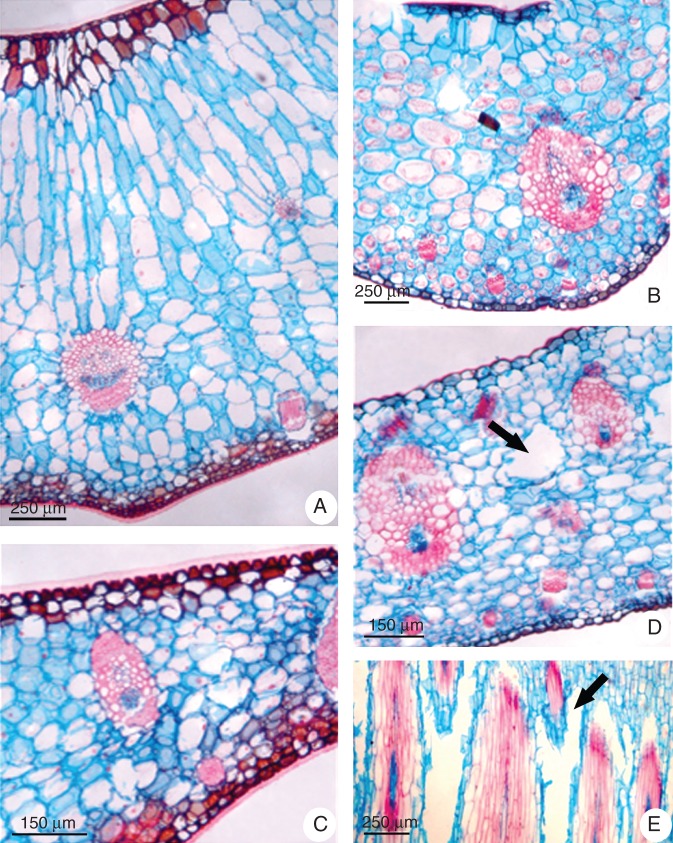
Cattleya walkeriana has succulent dorsiventral leaves with the palisade parenchyma formed by elongated cells showing several chloroplasts and large vacuoles (Fig. 1A), whereas Oncidium ‘Aloha’ has non-succulent leaves with intercellular air spaces in the thinner mesophyll and chlorenchyma cells smaller than in Cattleya (Fig. 1B). Moreover, Cattleya showed the epidermis covered by a conspicuously thicker cuticle (Fig. 1C) than that observed in Oncidium (Fig. 1D). While there was no noticeable aerenchyma in Cattleya leaves (Fig. 1A, C), this structure was detected in Oncidium mesophyll (Fig. 1D). Interestingly, in the leaf base this structure extended towards the pseudobulb at the limit of leaf and stem (Fig. 1E).
Fig. 1.
Comparative view of leaf morphology of (A, C) Cattleya walkeriana and (B, D, E) Oncidium ‘Aloha’. Light micrographs, transverse (A–D) and longitudinal (E) sections. (A, B) General view of transverse sections from the middle region of leaves showing the succulent morphology of Cattleya leaf (A) and the non-succulent constitution of Oncidium leaf (B). (C, D) Transverse sections from the leaf blade showing the epidermis of Cattleya covered by thicker cuticle on both surfaces (C) and the aerenchyma (arrow) between vascular bundles in Oncidium leaf (D), with detail of the aerenchyma near the pseudobulb (arrow) in a longitudinal section at the leaf base (E).
Pseudobulbs
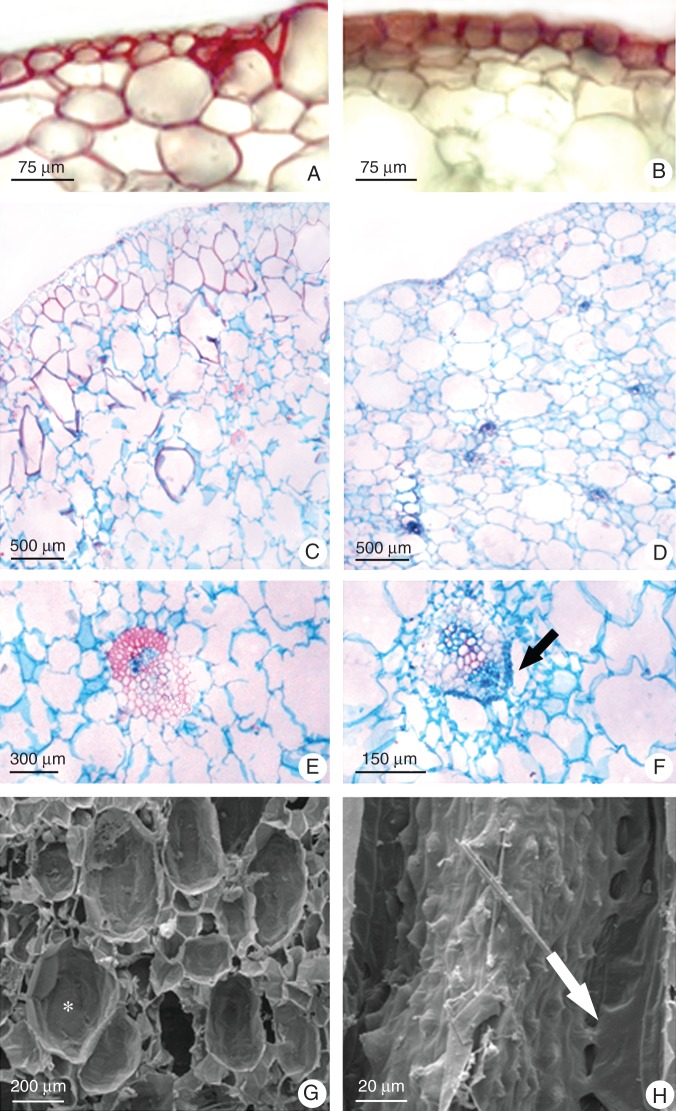
The pseudobulbs of both orchids were devoid of stomata (data not shown), with lignified epidermis (Fig. 2A, B), chlorenchyma without intercellular air spaces (Fig. 2C, D), large mucilage idioblasts (Fig. 2G) and vascular bundles scattered throughout the organ (Fig. 2C–F). However, the subepidermal parenchymatous cells of Cattleya pseudobulb were also lignified (Fig. 2C), while only Oncidium presented aerenchyma together with the vascular bundles adjacent to the phloem (Fig. 2F) throughout the organ length (Fig. 2H).
Fig. 2.
Comparative view of pseudobulb morphology of (A, C, E, G) Cattleya walkeriana and (B, D, F, H) Oncidium ‘Aloha’. (A, B) Lignin detection by phloroglucinol test (red stained). (C–F) Light microscopy. (C, D) Peripheral portion of the pseudobulb with vascular bundles spread through the organ. (E, F) The vascular bundle surrounded by chlorenchyma and mucilage idioblasts in Cattleya pseudobulb (E) and the presence of aerenchyma ducts (arrow) associated with the phloem of the Oncidium vascular bundle (F). (G, H) Scanning electron micrographs. Longitudinal sections of pseudobulbs showing mucilage idioblast (asterisk) in Cattleya (G) and the aerenchyma (arrow) associated with the Oncidium vascular bundle (H).
Roots
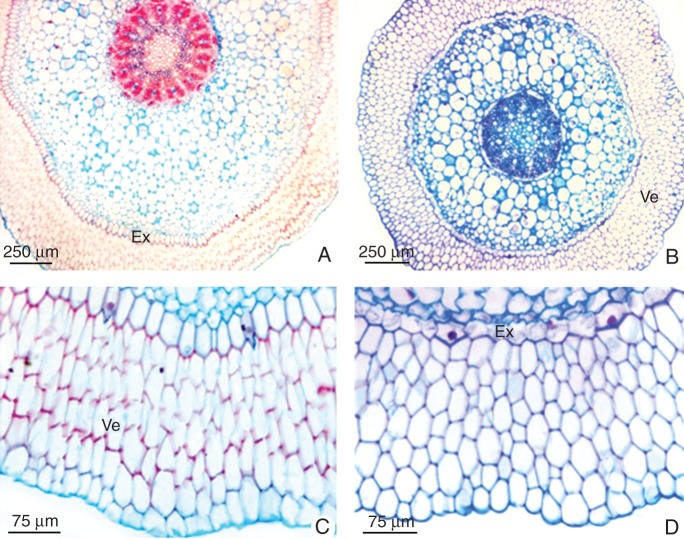
The histological features of roots of both orchids were relatively similar (Fig. 3); however, the cortex of Cattleya was wider than in Oncidium, mainly due to a higher number of chlorenchyma layers formed by smaller cells (Fig. 3A, B). In addition, the exodermal and the velamen cells of Cattleya roots seemed to be more strengthened than in Oncidium (Fig. 3C, D).
Fig. 3.
Transverse sections of the roots of (A, C) Cattleya walkeriana and (B, D) Oncidium ‘Aloha’. (A, B) General view of the roots. (C, D) Detail of the multilayered velamen (Ve) and the exodermis (Ex).
Drought-induced changes in relative water content
After 30 d of drought a significant reduction in the relative water content (RWC) was observed in all organs of Cattleya and Oncidium (Fig. 4). The water loss was more intense in roots of both orchids with an RWC decrease near 35 and 62 % in Cattleya and Oncidium, respectively. Besides, pseudobulbs of Cattleya and Oncidium lost only 10 and 25 % of their RWC, respectively, while leaves of both orchids showed RWC reduction of about 25 %.
Fig. 4.
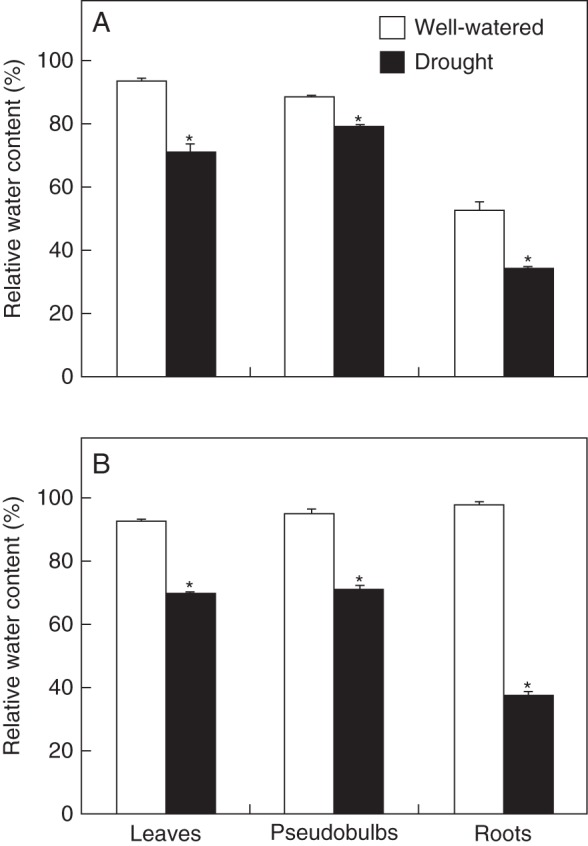
Relative water content in different organs of (A) Cattleya walkeriana and (B) Oncidium ‘Aloha’ after 1 month of well-watered or drought-exposed treatments (as indicated in key). Data are expressed as the mean ± s.e. An asterisk indicates a significant difference between treatments (P ≤ 0·05).
Drought-induced modulation of C3–CAM photosynthesis
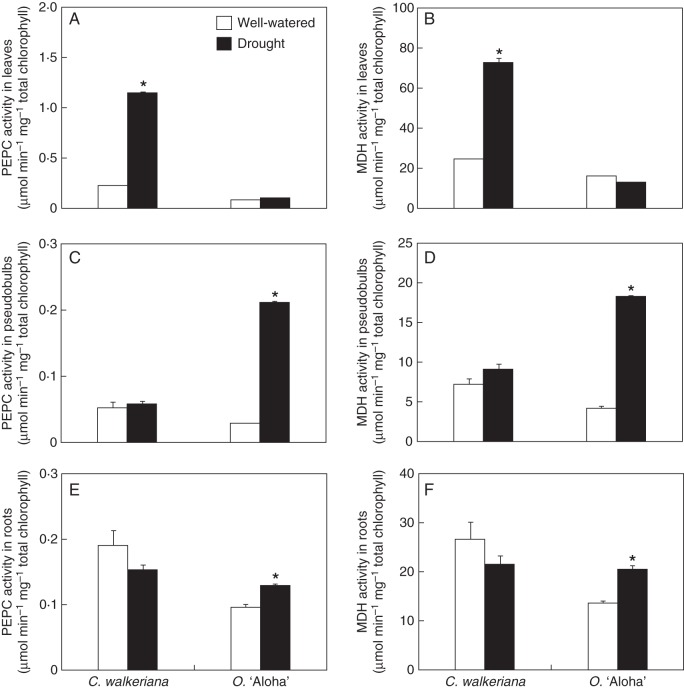
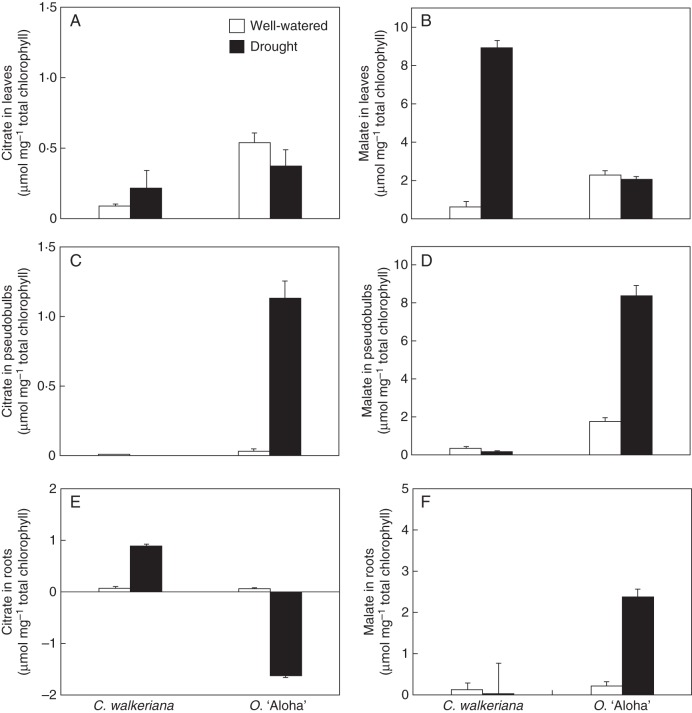
Both epiphytic orchids analysed presented distinct spatial responses in terms of CAM expression after 1 month of drought treatment (Figs 5 and 6). In Cattleya leaves, for instance, water deprivation induced a marked increase in PEPC and MDH activities (Fig. 5A, B), which was correlated with an expressive increment of nocturnal malate accumulation (Fig. 6B). By contrast, drought-treated Oncidium leaves did not show significant changes in PEPC/MDH activities (Fig. 5A, B), nor in night-time organic acid accumulation (Fig. 6A, B). Concurrently, day–night gas exchange analysis (Fig. 7) indicated that under well-watered conditions the Oncidium leaves carried out most of the atmospheric CO2 uptake during daytime, with very modest CO2 taken up at night. By contrast, when Oncidium plants were exposed to water shortage for 30 d, most of the daytime CO2 assimilation disappeared and only minor CO2 uptake remained in the middle of the afternoon (Fig. 7).
Fig. 5.
Activities of (A, C, E) PEPC and (B, D, F) MDH in leaves, pseudobulbs and roots of Cattleya walkeriana and Oncidium ‘Aloha’ after 1 month of well-watered or drought-exposed treatments (as indicated in key). Data are expressed as the mean ± s.e. An asterisk indicates a significant difference between treatments (P ≤ 0·05).
Fig. 6.
(A, C, E) Citrate and (B, D, F) malate nocturnal accumulation in leaves, pseudobulbs and roots of Cattleya walkeriana and Oncidium ‘Aloha’ after 1 month of well-watered or drought-exposed treatments (as indicated in key). Data are expressed as the differences between dawn and dusk values: organic acid mean = dawn mean–dusk mean. The standard error was calculated by the formula described by Popp et al. (2003): s.e. of the dawn–dusk difference = √[(s.e. dawn)2 + (s.e. dusk)2]. An asterisk indicates a significant difference between treatments (P ≤ 0·05).
Fig. 7.
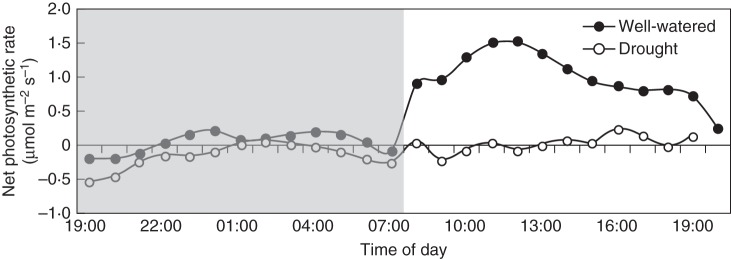
Diurnal pattern of CO2 assimilation in the apical leaf portion of Oncidium ‘Aloha’ plants maintained under well-watered conditions or exposed to 30 d of drought (as indicated in key). The shaded area indicates the dark period.
While Cattleya pseudobulbs showed no major changes in either CAM enzyme activities (Fig. 5C, D) or organic acid accumulation (Fig. 6C, D) after drought treatment, the Oncidium pseudobulbs displayed significant increases in both PEPC and MDH activities (Fig. 5C, D), associated with a remarkable increase in night-time malate and citrate accumulation (Fig. 6C, D). Oncidium roots displayed a significant increase in PEPC/MDH activities (Fig. 5E, F), considerable increase in nocturnal malate accumulation (Fig. 6F) and modest citrate loss during the night (Fig. 6E). Conversely, Cattleya roots showed fairly stable PEPC/MDH activities (Fig. 5E, F) and no nocturnal malate accumulation in both treatments (Fig. 6F). However, drought-treated Cattleya roots presented a slight nocturnal accumulation of citrate (Fig. 6E).
DISCUSSION
Relationship between organ morphology and water status
Thirty days of drought treatment was clearly enough to trigger significant water losses in virtually all organs of both orchids analyzed (Fig. 4). Comparing the overall pattern of water losses in the plant organs, Cattleya species (Fig. 4A) appeared to be relatively more resistant to water depletion than Oncidium (Fig. 4B). Interestingly, several morphological features were in agreement with the relatively more efficient control against water losses in Cattleya than in Oncidium plants (Figs 1–3). For instance, Cattleya roots showed a higher number of cell layers in the cortex and more lignified cell walls in both velamen and exodermis (Fig. 3A, C). The impermeability of Cattleya pseudobulbs was apparently increased due to a considerable deposition of lignin in their subepidermal parenchyma cell walls (Fig. 2A, C). Moreover, leaves of C. walkeriana presented lignified epidermis covered by a thicker cuticle and frequently lignified sclereids near stomata (Fig. 1A, C). The elevated density of sclereids in Cattleya leaves could be associated with higher hydraulic efficiency as these cellular structures can function as a hydraulic ‘shortcut’ through the mesophyll apoplast and as collapsible water storage elements that increase leaf capacitance (Brodribb et al., 2010).
Besides, the higher succulence showed by Cattleya leaves was mainly due to the presence of several chlorenchyma layers without noticeable intercellular air spaces (Fig. 1A). Hence, the larger vacuoles observed in the thicker chlorenchyma of this orchid represent a vastly abundant space for nocturnal acid accumulation, as reported in some surveys on Cattleya leaf photosynthesis (Nuerenbergk, 1963; Knauft and Arditti, 1969; Goh et al., 1977; Winter et al., 1983). Therefore, Cattleya showed a set of morphological characters that suggests ecological adaptations that would aid plants to reduce water loss. These structural features are typically observed in most thick-leaved epiphytic orchids, which, like C. walkeriana, are frequently found inhabiting harsher xerophytic environments (Knauft and Arditti, 1969; Avadhani et al., 1982).
Drought-induced modulation of C3–CAM photosynthesis in leaves
The drought treatment triggered significant increases in PEPC/MDH activities and nocturnal acid accumulation in Cattleya leaves (Figs 5A, B and 6B), which are essential metabolic features indicative of CAM expression (Borland et al., 2011). Despite this, no increase in these same parameters was observed in the thin leaves of Oncidium (Figs 5A, B and 6A, B). Previous reports have suggested that thick-leaved epiphytic orchids are commonly recognized as performing CAM photosynthesis in their succulent leaves (Avadhani and Arditti, 1981; Avadhani et al., 1982; Fu and Hew, 1982; Hew and Yong, 2004). Furthermore, the pronounced dark CO2 uptake and diurnal acidity rhythm characteristically found in Cattleya orchids are often linked to the succulent morphology of their leaves (Nuerenbergk, 1963; Knauft and Arditti, 1969).
However, the present results revealed that mature leaves of C. walkeriana, a typical thick-leaved orchid, can display a surprisingly high photosynthetic plasticity under distinct regimes of water availability (Figs 5A, B and 6A, B), which was comparable to the general pattern detected for the epiphytic bromeliad G. monostachia (Freschi et al., 2010b). Accordingly, well-watered leaves of C. walkeriana showed only mild diurnal fluctuations in both organic acid accumulation and PEPC/MDH activities, while the drought treatment induced the up-regulation of these metabolic parameters indicative of CAM expression (Figs 5A, B and 6A, B). These results reveal that even thick-leaved epiphytic orchids included in the genus Cattleya present relatively high plasticity in expressing CAM in their succulent leaves. In agreement with the present findings, leaves of the thick-leaved Phalaenopsis orchids showed up-regulation of CAM photosynthesis during plant ontogeny and in response to varied thermoperiodic conditions (Guo and Lee, 2006; Ping et al., 2010).
Although some nocturnal organic acid accumulation was observed in both well-watered and drought-treated Oncidium leaves (Fig. 6A, B), under well-watered conditions these leaves carried out most of the atmospheric CO2 uptake during daytime, which almost disappeared when Oncidium plants were exposed to water scarcity (Fig. 7). Considering these data, the thin leaves of O. ‘Aloha’ seemed to perform typical C3 photosynthesis even under drought conditions, which is in accordance with previous reports concerning the photosynthetic mode of leaves in the closely related hybrid Oncidium ‘Goldiana’ (Hew and Yong, 1994; Hew et al., 1996, 1998; Li et al., 2001, 2002).
The results presented here reinforce the idea that a higher degree of chlorenchyma succulence and reduced intercellular air space in leaves might be important for CAM operation (Nelson et al., 2005; Griffiths et al., 2008; Nelson and Sage, 2008; Borland et al., 2011). Hence, the set of morphological traits showed by Cattleya leaves might, to a certain extent, favour both water and carbon economy under drought by expressing CAM. On the other hand, Oncidium leaves might encounter some difficulties in maintaining a positive carbon balance under prolonged drought due to histological features that exhibit an apparently low efficiency in preventing water and CO2 losses. Therefore, these results suggest that photosynthetic activity in Oncidium leaves under water scarcity might be, at least in part, dependent on the non-leaf organs, such as pseudobulbs and roots.
Drought-induced modulation of C3–CAM photosynthesis in roots
Despite the small amount of citrate accumulation during the night (Fig. 6E), aerial roots of the drought-treated Cattleya plants showed an apparent lack of CAM expression (Figs 5E, F and 6F). Therefore, such photosynthetic compartmentalization found among organs of the drought-treated Cattleya (CAM in leaves versus C3 in roots) is in agreement with recent research which has shown that the presence of CAM in leaves of epiphytic orchids did not ensure that their aerial roots would perform the same photosynthetic pathway (Martin et al., 2010). Conversely, the drought-treated roots of Oncidium presented a significant increase in nocturnal malate accumulation (Fig. 6F) and also showed a significant increase in PEPC/MDH activities (Fig. 5E, F), indicating that induction, or at least up-regulation, of CAM expression was triggered in aerial roots of this orchid by water limitation. As far as we know, the data obtained with Oncidium might be the first demonstration regarding the occurrence of inducible CAM in aerial roots of an orchid performing typical C3 photosynthesis in its leaves.
The view that aerial roots of leafy orchids possess the photosynthetic apparatus for CO2 fixation but, in general, are not considered sufficiently autotrophic to maintain themselves (Ho et al., 1983; Hew et al., 1984) suggests a more localized role for the presence of CAM in aerial roots of Oncidium under water deficit. However, the larger chlorophyll-containing cells in the Oncidium cortex could provide the photosynthetic machinery and the vacuolar space required for nocturnally accumulating organic acids derived from CO2 fixation through PEPC activity. In support of this hypothesis, autotrophic roots of some leafless epiphytic orchids, although lacking stomata, represent important photosynthetic organs for the plant. The uptake and fixation of CO2 by these autotrophic roots can occur nocturnally due to the presence of a thinner velamen and larger volume of cortical intracellular space when compared with the same structures in aerial roots of most leafy orchids (Benzing et al., 1983; Winter et al., 1983; Cockburn et al., 1985). Accordingly, studies regarding the relationship between respiration and CO2 fixation by aerial roots of Aranda orchids have suggested that its thick velamen hampers CO2 uptake from the atmosphere, while the extent of this effect might depend on velamen thickness (Hew et al., 1991).
Drought-induced modulation of C3–CAM photosynthesis in pseudobulbs
Virtually no nocturnal organic acid accumulation was detected in both well-watered and drought-treated Cattleya pseudobulbs (Fig. 6C, D), which was supported by no changes in PEPC/MDH activities (Fig. 5C, D), thus indicating the absence of CAM expression in pseudobulbs of C. walkeriana. Alternatively, the drought treatment triggered a remarkable increase in night-time organic acid accumulation in Oncidium pseudobulbs, which was followed by a parallel rise in the activities of both PEPC and MDH (Figs 5C, D and 6C, D), thereby implying that Oncidium pseudobulbs can be induced to perform CAM photosynthesis depending on environmental conditions.
As most orchid pseudobulbs have a hermetic structure in which the entire organ is covered with thick cuticle and is devoid of stomata, the main explanation for the nocturnal organic acid accumulation in this organ has been based on recycling respiratory CO2 generated by the highly packed pseudobulb parenchyma (Hew and Yong, 1994; Ng and Hew, 2000). Contrary to the general structural organization observed in ground tissue of Oncidium, the outer portion of Cattleya pseudobulbs showed a considerable number of dead cells with thickened, lignified walls scattered among assimilatory cells, creating a sclerenchymatous boundary around the periphery of the ground tissue (Fig. 2C). This type of cellular arrangement has been reported for other orchid species (Withner, 1974; Stern and Morris, 1992; Holtzmeier et al., 1998), and, for C. walkeriana, it might restrict the amount of living cells with the metabolic requirements for modulating the photosynthetic pathway.
Note that Oncidium pseudobulbs, in addition to malate, also nocturnally accumulated citrate, but the amounts were lower than those found for malate (Fig. 6C, D). Although the putative functional advantage of performing CAM with malate and/or citrate is not fully understood, some evidence has indicated that nocturnal accumulation of citrate might be more favourable than malate under certain environmental constraints, such as drought associated with high-irradiance stress (Lüttge, 2002, 2006). Besides, the higher energy demand for citrate cycling in the light period can contribute to energy dissipation and cellular redox balance, thus acting as a protective mechanism against photoinhibition and photodestruction (Kornas et al., 2009; Sun and Hong, 2011).
Hypothetical model for drought-induced C3–CAM compartmentalization in Oncidium
Previous approaches to the photosynthetic metabolism of Oncidium ‘Goldiana’ cast some persistent doubts regarding the actual physiological dynamics behind the complex interdependency observed between leaves and pseudobulbs of this thin-leaved epiphytic orchid. These studies revealed an apparent inconsistency between data obtained by gas exchange and radioactive tracer experiments demonstrating that photosynthates in leaves were somehow transported to the pseudobulb in the first instance and then re-distributed within the shoot organs (Yong and Hew, 1995a, b). This dilemma, which has remained unclear until now, has been attributed to possible unique patterns of photoassimilate partitioning in tropical orchids which might result from complex vascular connections between source and sink organs (Hew and Yong, 1994; Yong and Hew, 1995a; Hew et al., 1996; Ng and Hew, 2000).
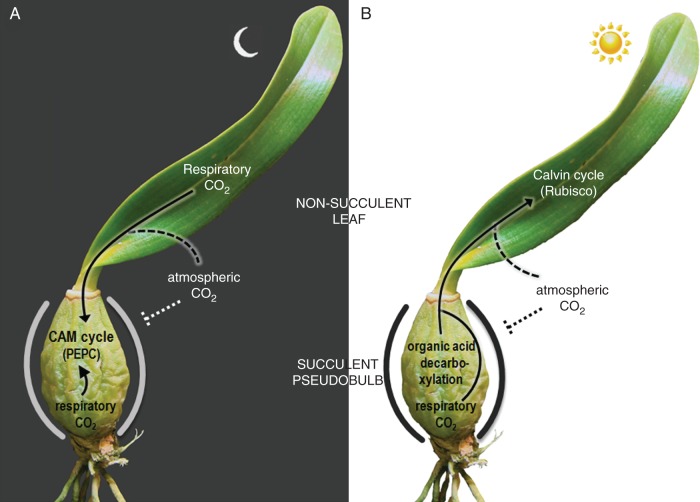
Accordingly, the present results showed that in Oncidium ‘Aloha’ pseudobulbs all the vascular bundles were associated with an aerenchyma (Fig. 2F), which was present throughout the entire length of the pseudobulb (Fig. 2H) that was able to express CAM under drought conditions (Figs 5C, D and 6C, D). Intriguingly, these aerenchyma ducts along the pseudobulb were connected with the air spaces present at the leaf base of Oncidium (Fig. 1E), thus forming free pathways for gas exchange between leaves and pseudobulbs. Equivalent structural organization has been described for some orchids, including species from the subtribe Oncidiinae, where the aerenchyma (termed ‘lacunae’) is also associated with the pseudobulb phloem (Moreau, 1913; Withner, 1974; Holtzmeier et al., 1998; Stern and Carlsward, 2006; Aybeke et al., 2010).
Therefore, these findings make it possible to suggest a hypothetical model for the photosynthetic compartmentalization found among organs of the drought-treated O. ‘Aloha’ orchid (Fig. 8). Based on this proposition, at least some of the production of the organic acids in this organ might possibly come from the carboxylation of CO2 originated by the nocturnal respiration of the leaf mesophyll cells, or even from the atmosphere, if some leaf stomata are open at night. By contrast, during the day, if leaf stomata remain closed due to a certain limitation in water supply or other environmental stress, the decarboxylation of organic acids in chlorenchyma cells of the pseudobulbs could possibly provide CO2 for leaf mesophyll cells through the aerenchyma ducts. If this is the case, the site of organic acid accumulation may be transferred from the non-succulent leaf mesophyll of Oncidium to the larger vacuoles of the chlorenchyma cells in the succulent pseudobulb of this orchid. Therefore, in this case, we may have spatial separation between the site of night-time carboxylation via PEPC (pseudobulbs) and the site of daytime carboxylation via Rubisco (leaves). Perhaps, the limited capacity for nocturnal acid storage in the thin leaf mesophyll of Oncidium could be surpassed by transferring CAM expression to the pseudobulbs, which besides being succulent are also devoid of stomata, thus limiting any eventual loss of CO2 to the atmosphere.
Fig. 8.
Schematic representation of the general model suggested for the photosynthetic compartmentalization among organs of the drought-treated Oncidium ‘Aloha’ orchid (C3 in leaves versus CAM in non-leaf organs) during night (A) and daytime (B). The model is based on the presence of aerenchyma ducts connecting the succulent pseudobulb with the mesophyll of non-succulent leaves (solid arrows) and, consequently, with the atmosphere (dashed lines). The sharper lines surrounding the pseudobulb indicate the hermetic feature of this organ which eliminates direct atmospheric carbon fixation. Details are given in the text.
This might help to clarify aspects that still persist regarding the complex dynamics that coordinate the photosynthetic interdependency observed between leaves and pseudobulbs of Oncidium orchids. However, from the perspective of this evidence, questions remain about whether we should consider Oncidium plants as performing C3 photosynthesis (when taking into consideration just the data obtained from the leaves), or a facultative CAM plant (by considering the drought-induced up-regulation of CAM in the non-leaf organs), or even as an example of a special mode of inducible CAM photosynthesis in which the day–night acid cycle typical of CAM (in the pseudobulbs) is spatially separated from the Calvin cycle (in the leaf mesophyll).
Concluding remarks
Here we demonstrate that water availability is a powerful signal capable of modulating CAM expression in an organ/tissue-compartmented manner in both the thick-leaved (C. walkeriana) and thin-leaved (O. ‘Aloha’) epiphytic orchids studied. Although belonging to an orchid genus classically considered as performing C3 photosynthesis, Oncidium plants under drought seemed to express facultative CAM in its roots and pseudobulbs but not in its leaves; the drought-induced CAM expression in these organs might compensate for the lack of capacity to perform CAM in its thin leaves. On the other hand, C. walkeriana, which is considered a constitutive CAM orchid, has shown a clear drought-induced up-regulation of CAM in its thick leaves but not in its non-leaf organs. As distinct regions of the same orchid could perform different photosynthetic pathways and variable degrees of CAM expression depending on water availability, more attention should be given to this during future studies about the abundance of CAM plants in a given plant family, habitat and ecosystem. The data presented highlight the great importance of studying the range of CAM expression/modulation in specific plant tissues while taking into consideration the physiological responses under different environmental conditions and/or developmental phases.
ACKNOWLEDGMENTS
We thank the Colibri Orquídeas (São Lourenço da Serra, SP, Brazil – www.colibriorquidea.com) and the LC Orquídeas (Av. Arquimedes, 1074, 13211-840, Jundiaí, SP, Brazil) for supplying the plants used in this study. We also thank Maxuel de Oliveira Andrade for invaluable technical assistance with organic acid quantification, and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo), CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) for providing financial support.
LITERATURE CITED
- Amorós A, Zapata P, Pretel MT, Botella MA, Serrano M. Physico-chemical and physiological changes during fruit development and ripening of five loquat (Eriobotrya japonica Lindl.) cultivars. Food Science and Technology International. 2003;9:43–51. [Google Scholar]
- Ando T, Ogawa M. Photosynthesis of leaf blades in Laelia anceps Lindl. is influenced by irradiation of pseudobulb. Photosynthetica. 1987;21:588–590. [Google Scholar]
- Arditti J. Aspects of orchid physiology. In: Woolhouse HW, editor. Advances in botanical research. Vol. 7. London: Academic Press; 1979. pp. 421–655. [Google Scholar]
- Aschan G, Pfanz H. Non-foliar photosynthesis – a strategy of additional carbon acquisition. Flora. 2003;198:81–97. [Google Scholar]
- Avadhani PN, Arditti J. Carbon fixation in orchids. In: Lawler L, Kerr RD, editors. Proceedings of the Orchid Symposium. Sydney: Harbour Press; 1981. pp. 79–85. [Google Scholar]
- Avadhani PN, Goh CJ, Rao AN, Arditti J. Carbon fixation in orchids. In: Arditti J, editor. Orchid biology, reviews and perspectives. Vol. 2. Ithaca: Cornell University Press; 1982. pp. 173–193. [Google Scholar]
- Aybeke M, Sezik E, Olgun G. Vegetative anatomy of some Ophrys, Orchis and Dactylorhiza (Orchidaceae) taxa in Trakya region of Turkey. Flora. 2010;205:73–89. [Google Scholar]
- Barrs HD, Weatherley PE. A re-examination of relative turgidity technique for estimating water deficits in leaves. Australian Journal of Biological Sciences. 1962;15:413–428. [Google Scholar]
- Benzing DH. The evolution of epiphytism. In: Lüttge U, editor. Vascular plants as epiphytes: evolution and ecophysiology. Berlin: Springer-Verlag; 1989. pp. 15–41. [Google Scholar]
- Benzing DH. Vascular epiphytes: general biology and related biota. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Benzing DH, Ott DW. Vegetative reduction in epiphytic Bromeliaceae and Orchidaceae: its origin and significance. Biotropica. 1981;13:131–140. [Google Scholar]
- Benzing DH, Friedman WE, Peterson G, Renfrow A. Shootlessness, velamentous roots, and the pre-eminence of Orchidaceae in the epiphytic biotope. American Journal of Botany. 1983;70:121–133. doi: 10.1002/j.1537-2197.1983.tb12440.x. [DOI] [PubMed] [Google Scholar]
- Borland AM, Zambrano VAB, Ceusters J, Shorrock K. The photosynthetic plasticity of crassulacean acid metabolism: an evolutionary innovation for sustainable productivity in a changing world. New Phytologist. 2011;191:619–633. doi: 10.1111/j.1469-8137.2011.03781.x. [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Feild TS, Sack L. Viewing leaf structure and evolution from a hydraulic perspective. Functional Plant Biology. 2010;37:488–498. [Google Scholar]
- Cockburn W, Goh CJ, Avadhani PN. Photosynthetic carbon assimilation in a shootless orchid, Chiloschista usneoides (DON) LDL: a variant on crassulacean acid metabolism. Plant Physiology. 1985;77:83–86. doi: 10.1104/pp.77.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y-Y, Pandey DM, Hahn E-J, Paek K-Y. Effect of drought on physiological aspects of crassulacean acid metabolism in Doritaenopsis. Plant Science. 2004;167:1219–1226. [Google Scholar]
- Cushman JC. Crassulacean acid metabolism: a plastic photosynthetic adaptation to arid environments. Plant Physiology. 2001;4:1439–1448. [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K. Crassulacean acid metabolism: plastic, fantastic. Journal of Experimental Botany. 2002;53:1–12. doi: 10.1093/jexbot/53.369.569. [DOI] [PubMed] [Google Scholar]
- Dressler RL. The orchids: natural history and classification. Cambridge: Harvard University Press; 1981. [Google Scholar]
- Dressler RL. Phylogeny and classification of the orchid family. Portland: Dioscorides Press; 1993. [Google Scholar]
- Freschi L, Rodrigues MA, Domingues DS, et al. Nitric oxide mediates the hormonal control of crassulacean acid metabolism expression in young pineapple plants. Plant Physiology. 2010;152:1971–1985. doi: 10.1104/pp.109.151613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freschi L, Takahashi CA, Cambuí CA, et al. Specific leaf areas of the tank bromeliad Guzmania monostachia perform distinct functions in response to water shortage. Journal of Plant Physiology. 2010;167:526–533. doi: 10.1016/j.jplph.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Fu CF, Hew CS. Crassulacean acid metabolism in orchids under water stress. Botanical Gazette. 1982;143:294–297. [Google Scholar]
- Gerlach D. Botanische Mikrotechnik: eine Einführung. Stuttgart: Georg Thieme Verlag; 1984. [Google Scholar]
- Goh CJ, Kluge M. Gas exchange and water relations in epiphytic orchids. In: Lüttge U, editor. Vascular plants as epiphytes: evolution and ecophysiology. Berlin: Springer-Verlag; 1989. pp. 139–166. [Google Scholar]
- Goh CJ, Avadhani PN, Loh CS, Hanegraaf C, Arditti J. Diurnal stomatal and acidity rhythms in orchid leaves. New Phytologist. 1977;78:365–372. [Google Scholar]
- Goh CJ, Arditti J, Avadhani PN. Carbon fixation in orchid aerial roots. New Phytologist. 1983;95:367–374. [Google Scholar]
- Gravendeel B, Smithson A, Slik FJW, Schuiteman A. Epiphytism and pollinator specialization: drivers for orchid diversity? Philosophical Transactions of the Royal Society B. 2004;359:1523–1535. doi: 10.1098/rstb.2004.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths H. Carbon dioxide concentrating mechanisms and the evolution of CAM in vascular epiphytes. In: Lüttge U, editor. Vascular plants as epiphytes: evolution and ecophysiology. Berlin: Springer-Verlag; 1989. pp. 42–86. [Google Scholar]
- Griffiths H, Robe WE, Girnus J, Maxwell K. Leaf succulence determines the interplay between carboxylase systems and light use during crassulacean acid metabolism in Kalanchöe species. Journal of Experimental Botany. 2008;59:1851–1861. doi: 10.1093/jxb/ern085. [DOI] [PubMed] [Google Scholar]
- Guo W-J, Lee N. Effect of leaf and plant age, and day/night temperature on the net CO2 uptake in Phalaenopsis amabilis var. formosa. Journal of the American Society for Horticultural Science. 2006;131:320–326. [Google Scholar]
- Hasegawa PN, Faria AF, Mercadante AZ, et al. Chemical composition of five loquat cultivars planted in Brazil. Ciência e Tecnologia de Alimentos. 2010;30:552–559. [Google Scholar]
- Herrera A. Crassulacean acid metabolism and fitness under water deficit stress: if not for carbon gain, what is facultative CAM good for? Annals of Botany. 2009;103:645–653. doi: 10.1093/aob/mcn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew CS, Yong JWH. Growth and photosynthesis of Oncidium Goldiana. Journal of Horticultural Science. 1994;69:809–819. [Google Scholar]
- Hew CS, Yong JWH. The physiology of tropical orchids in relation to the industry. Singapore: World Scientific; 2004. [Google Scholar]
- Hew CS, Ng YW, Wong SC, Yeoh HH, Ho KK. Carbon dioxide fixation in orchid aerial roots. Physiologia Plantarum. 1984;60:154–158. [Google Scholar]
- Hew CS, Ye QS, Pan RC. Relation of respiration to CO2 fixation by Aranda orchid roots. Environmental and Experimental Botany. 1991;31:327–331. [Google Scholar]
- Hew CS, Ng CKY, Gouk SS, Yong JWH, Wong SC. Variation in δ13C values for different plant parts of an Oncidium orchid. Photosynthetica. 1996;32:135–139. [Google Scholar]
- Hew CS, Soh WP, Ng CKY. Variation in photosynthetic characteristics along the leaf blade of Oncidium Goldiana, a C3 tropical epiphytic orchid hybrid. International Journal of Plant Sciences. 1998;159:116–120. [Google Scholar]
- Ho K-K, Yeoh H-H, Hew C-S. The presence of photosynthetic machinery in aerial roots of leafy orchids. Plant and Cell Physiology. 1983;24:1317–1321. [Google Scholar]
- Holtzmeier MA, Stern WL, Judd WS. Comparative anatomy and systematics of Senghas's cushion species of Maxillaria (Orchidaceae) Botanical Journal of the Linnean Society. 1998;127:43–82. [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw-Hill; 1940. [Google Scholar]
- Kerbauy GB, Takahashi CA, Lopez AM, et al. Crassulacean acid metabolism in epiphytic orchids: current knowledge, future perspectives. In: Najafpour MM, editor. Applied photosynthesis. Rijeka: InTech; 2012. pp. 81–104. [Google Scholar]
- Knauft RL, Arditti J. Partial identification of dark 14CO2 fixation products in leaves of Cattleya (Orchidaceae) New Phytologist. 1969;68:657–661. [Google Scholar]
- Kornas A, Fuscher-Schliebs E, Lüttge U, Miszalski Z. Adaptation of the CAM plant Clusia alata to light stress: metabolic responses. Journal of Plant Physiology. 2009;166:1914–1922. doi: 10.1016/j.jplph.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Kress WJ. The systematic distribution of vascular epiphytes: an update. Selbyana. 1986;9:2–22. [Google Scholar]
- Lacerda KG. Amazon discovery of new species and extinction. In: Shirasaea T, editor. Brazilian orchids. Tokyo: Sodo Publishing Company; 1995. pp. 10–123. [Google Scholar]
- Li CR, Sun WQ, Hew CS. Up-regulation of sucrose metabolizing enzymes in Oncidium Goldiana grown under elevated carbon dioxide. Physiologia Plantarum. 2001;113:15–22. [Google Scholar]
- Li CR, Heng YH, Hew CS. Responses of Rubisco and sucrose-metabolizing enzymes to different CO2 in a C3 tropical epiphytic orchid Oncidium Goldiana. Plant Science. 2002;163:313–320. [Google Scholar]
- Lillie RD. Histopathologic technique and practical histochemistry. New York: McGraw-Hill; 1965. [Google Scholar]
- Lüttge U. CO2-concentrating: consequences in crassulacean acid metabolism. Journal of Experimental Botany. 2002;53:2131–2142. doi: 10.1093/jxb/erf081. [DOI] [PubMed] [Google Scholar]
- Lüttge U. Ecophysiology of crassulacean acid metabolism (CAM) Annals of Botany. 2004;93:629–652. doi: 10.1093/aob/mch087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U. Photosynthetic flexibility and ecophysiological plasticity: questions and lessons from Clusia, the only CAM tree, in the neotropics. New Phytologist. 2006;171:7–25. doi: 10.1111/j.1469-8137.2006.01755.x. [DOI] [PubMed] [Google Scholar]
- Martin CE, Mas EJ, Lu C, Ong BL. The photosynthetic pathway of the roots of twelve epiphytic orchids with CAM leaves. Photosynthetica. 2010;48:42–50. [Google Scholar]
- Moreau L. Etude anatomique des Orchidées à pseudo-bulbes des pays chauds et de quelques autres espèces tropicales de plantes à tubercules. Revue Générale de Botanique. 1913;25:503–548. [Google Scholar]
- Moreira ASFP, Filho JPL, Zotz G, Isaias RMS. Anatomy and photosynthetic parameters of roots and leaves of two shade-adapted orchids, Dichaea cogniauxiana Shltr. and Epidendrum secundum Jacq. Flora. 2009;204:604–611. [Google Scholar]
- Motomura H, Ueno O, Kagawa A, Yukawa T. Carbon isotope ratios and the variation in the diurnal pattern of malate accumulation in aerial roots of CAM species of Phalaenopsis (Orchidaceae) Photosynthetica. 2008;46:531–536. [Google Scholar]
- Neales TF, Hew CS. Two types of carbon fixation in tropical orchids. Planta. 1975;123:303–306. doi: 10.1007/BF00390710. [DOI] [PubMed] [Google Scholar]
- Nelson EA, Sage R. Functional constraints of CAM leaf anatomy: tight cell packing is associated with increased CAM function across a gradient of CAM expression. Journal of Experimental Botany. 2008;59:1841–1850. doi: 10.1093/jxb/erm346. [DOI] [PubMed] [Google Scholar]
- Nelson EA, Sage TL, Sage RF. Functional leaf anatomy of plants with crassulacean acid metabolism. Functional Plant Biology. 2005;32:409–419. doi: 10.1071/FP04195. [DOI] [PubMed] [Google Scholar]
- Ng CKY, Hew CS. Orchid pseudobulbs – ‘false’ bulbs with a genuine importance in orchid growth and survival! Scientia Horticulturae. 2000;83:165–172. [Google Scholar]
- Nuerenbergk EL. On the CO2 metabolism of orchids and its ecological aspects. In: Chon YB, editor. Proceedings of the Fourth World Orchid Conference. Singapore: Straits Times Press; 1963. pp. 158–169. [Google Scholar]
- Pearse AGE. Histochemistry: theoretical and applied. Analytical technology. Vol. 2. Edinburgh: Churchill Livingstone; 1985. [Google Scholar]
- Ping CY, Lee Y-I, Lin TS, Yang WJ, Lee GC. Crassulacean acid metabolism in Phalaenopsis aphrodite var. formosa during different developmental stages. Acta Horticulturae. 2010;878:71–77. [Google Scholar]
- Popp M, Janett H-P, Lüttge U, Medina E. Metabolite gradients and carbohydrate translocation in rosette leaves of CAM and C3 bromeliads. New Phytologist. 2003;157:649–656. doi: 10.1046/j.1469-8137.2003.00683.x. [DOI] [PubMed] [Google Scholar]
- Pridgeon AM. Anatomical adaptations in Orchidaceae. Lindleyana. 1986;1:90–101. [Google Scholar]
- Sheehan T, Sheehan M. An illustrated survey of orchid genera. Oregon: Timber Press; 1994. [Google Scholar]
- Silvera K, Santiago LS, Winter K. Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection of weak and strong modes. Functional Plant Biology. 2005;32:397–407. doi: 10.1071/FP04179. [DOI] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K. Crassulacean acid metabolism and epiphytism linked to adaptive radiations in the Orchidaceae. Plant Physiology. 2009;149:1838–1847. doi: 10.1104/pp.108.132555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams NH, Winter K, Cushman JC. Evolution along the crassulacean acid metabolism continuum. Functional Plant Biology. 2010;37:995–1010. [Google Scholar]
- Silvera K, Santiago LS, Cushman JC, Winter K. The incidence of crassulacean acid metabolism in Orchidaceae derived from carbon isotope ratios: a checklist of the flora of Panama and Costa Rica. Botanical Journal of the Linnean Society. 2010b;163:194–222. [Google Scholar]
- Sinclair R. Water relations in orchids. In: Arditti J, editor. Orchid biology: reviews and perspectives. Vol. 5. Portland: Timber Press; 1990. pp. 63–119. [Google Scholar]
- Stern WL, Carlsward BS. Comparative vegetative anatomy and systematics of the Oncidiinae (Maxillarieae, Orchidaceae) Botanical Journal of the Linnean Society. 2006;152:91–107. [Google Scholar]
- Stern WL, Morris MW. Vegetative anatomy of Stanhopea (Orchidaceae) with special reference to pseudobulb water-storage cells. Lindleyana. 1992;7:34–53. [Google Scholar]
- Sun Y-L, Hong S-K. Effects of citric acid as an important component of the responses to saline and alkaline stress in the halophyte Leymus chinensis (Trin.) Plant Growth Regulation. 2011;64:129–139. [Google Scholar]
- Williams NH, Chase MW, Fulcher T, Whitten WM. Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscription of Cyrtochilum, Erycina, Otoglossum, and Trichocentrum and a new genus (Orchidaceae) Lindleyana. 2001;16:113–139. [Google Scholar]
- Winter K, Wallace BJ, Stocker GC, Roksandic Z. Crassulacean acid metabolism in Australian vascular epiphytes and some related species. Oecologia. 1983;57:129–141. doi: 10.1007/BF00379570. [DOI] [PubMed] [Google Scholar]
- Winter K, Garcia M, Holtum JAM. On the nature of facultative and constitutive CAM: environmental and developmental control of CAM expression during early growth of Clusia, Kalanchoë, and Opuntia. Journal of Experimental Botany. 2008;59:1829–1840. doi: 10.1093/jxb/ern080. [DOI] [PubMed] [Google Scholar]
- Withner C. The orchids: scientific studies. New York: Wiley; 1974. [Google Scholar]
- Wu F-H, Chan M-T, Liao D-C, et al. Complete chloroplast genome of Oncidium Gower Ramsey and evaluation of molecular markers for identification and breeding in Oncidiinae. BioMed Central Plant Biology. 2010;10:68–79. doi: 10.1186/1471-2229-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong JWH, Hew CS. The importance of photoassimilate contribution from the current shoot and connected back shoots to inflorescence size in the thin-leaved sympodial orchid Oncidium Goldiana. International Journal of Plant Sciences. 1995a;156:450–459. [Google Scholar]
- Yong JWH, Hew CS. Partitioning of 14C assimilates between sources and sinks during different growth stages in the sympodial thin-leaved orchid Oncidium Goldiana. International Journal of Plant Sciences. 1995;156:188–196. [Google Scholar]
- Zotz G, Hietz P. The physiological ecology of vascular epiphytes: current knowledge, open questions. Journal of Experimental Botany. 2001;52:2067–2078. doi: 10.1093/jexbot/52.364.2067. [DOI] [PubMed] [Google Scholar]