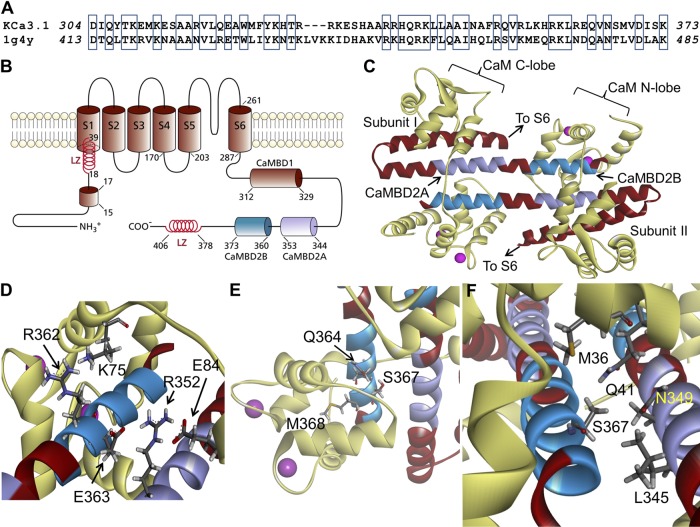
Figure 1.
Structural model obtained for the Ca2+-CaM N-lobe–KCa3.1 complex. (A) Sequence alignment between the KCa3.1 segment extending from D304 to K373 and the corresponding sequence used as template for homology modeling based on the 1G4Y (Protein Data Bank; SK2) structure. Alignment was generated by MUSTER. Identical residues are identified in open boxes for an overall sequence identity of 45%. (B) Membrane topology of KCa3.1. KCa3.1 is a tetrameric protein, with each monomer organized in six transmembrane segments plus a pore region between segments 5 and 6. The channel Ca2+ sensitivity is conferred by CaM, with the CaM C-lobe constitutively bound to the 312–329 segment of the channel C-terminal region (CaMBD1). The Ca2+-dependent binding of the CaM to the channel involves the segment 344 to 353 (CaMBD2A) and a stretch of 13 amino acids from 360 to 373 (CaMBD2B). In the N terminus, a 15RKR17 motif has been identified as being potentially involved in N terminus/C terminus coupling. Also illustrated are two leucine zipper (LZ) domains important for channel expression at the membrane. (C) Ribbon representation of the Ca2+-CaM N-lobe–KCa3.1 complex. The initial model was generated with MODELLER using the 1G4Y (Protein Data Bank) crystal structure as template. Each KCa3.1 subunit comprises the amino acids D304 to K373, with the CaM represented in light yellow. The CaMBD2B (K360-K373) is colored cyan, and the CaMBD2A is represented in purple. (D) Close-up of the complex, illustrating residue R362 of KCa3.1 in close proximity of K75 of CaM, and R352 facing both E84 of CaM and E363 on the adjacent KCa3.1 subunit. (E) Illustration of M368 projecting inside the CaM N-lobe together with S367 and Q364. This figure also includes a space-filling representation of two calcium ions as pink-colored spheres. (F) Detailed representation of the interaction domain of S367, with S367 contributing to the intersubunit interface, which includes residues L345 and N349 of the adjacent subunit, and to the CaM N-lobe–CaMBD2B interface with residues M36, L39 (not depicted), and Q41 of CaM. Visualization was generated with Discovery Studio (Accelrys, Inc.).