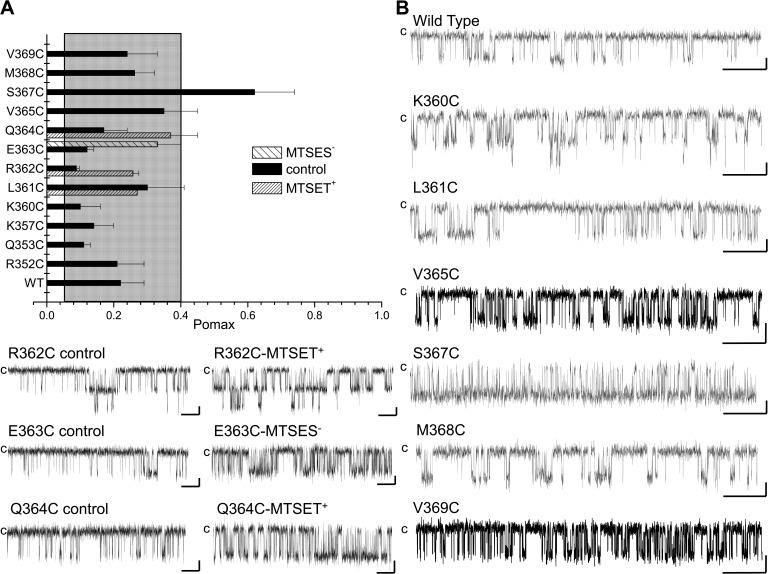
Figure 6.
Effect of mutating residues of the CaMBD2 on Pomax. (A) Bar graph summarizing measurements of Pomax, the channel open probability at saturating Ca2+ concentration (25 µM), for channel mutants generated by substituting individual residues along CaMBD2 to a Cys. Values not included in the filled area refer to Pomax for which P < 0.005 relative to WT (Pomax (WT) ± 2.5 SD). A strong increase was seen with the S367C mutant, with Pomax of 0.62 ± 0.12 (n = 5) compared with 0.22 ± 0.07 (n = 8) for WT KCa3.1. (B) Examples of inside-out current traces obtained for the WT, K360C, L361C, R362C, E363C, Q364C, V365C, S367C, M368C, and V369C mutant channels expressed in Xenopus oocytes. Dwell-time analyses showed that the increase in Pomax observed with the S367C mutant resulted in an increase of the channel mean open time from 7 ± 2 ms (n = 5) for WT to 25 ± 7 ms (n = 4) for S367C, and a decrease of the channel mean closed time from 30 ± 15 ms (n = 5) for WT to 5 ± 1 ms (n = 4) for S367C mutant. Also included are single-channel traces illustrating the action of MTSES− or MTSET+ on the R362C, E363C, and Q364C mutants, respectively. These results confirmed that residues identified from SASA calculations as being either buried (Q364) or exposed (R362) can be modified by MTS reagents, suggesting that the targeted Cys residues were accessible to water. Current recordings were performed in symmetrical 200-mM K2SO4 solutions at an internal Ca2+ concentration of 25 µM, with 100 mV applied in the patch electrode. Label “c” refers to the channel closed state. Bars, 2 pA and 500 ms.