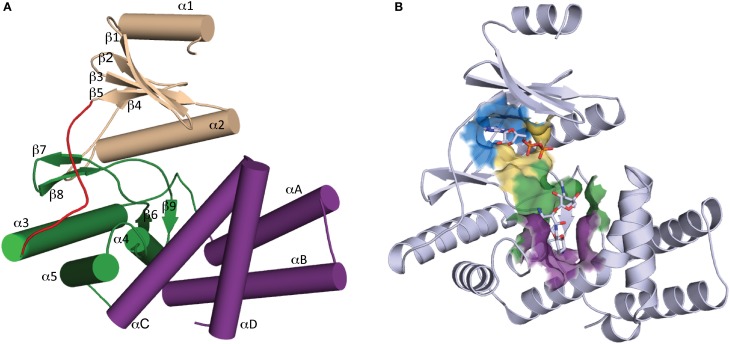
Figure 2.
Overall structure of APH. (A) Secondary structure elements of a typical APH enzyme. The N-terminal lobe is colored tan, the hinge region is colored red, the core and helical subdomains in the C-terminal lobe are colored green and purple, respectively. (B) Cartoon representation of APH(2″)-IIa with ADP and gentamicin C1a in stick representation. The location of the active site is highlighted by a surface where the nucleoside pocket is colored blue, the triphosphate pocket is colored yellow, the catalytic pocket is in green, and the specificity pocket is in purple.