Table 1.
Domino synthesis of indoles 4 under MWa
| Entry | Product | R or R′ | Time/min | Yieldb/% |
|---|---|---|---|---|
| 1 |
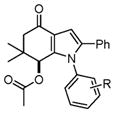 4a–4d |
4a, H (1a) | 18 | 85 |
| 2 | 4b, 4-Fluoro (1b) | 20 | 81 | |
| 3 | 4c, 4-Bromo (1d) | 22 | 82 | |
| 4 | 4d, 4-Methyl (1e) | 16 | 88 | |
| 5 |
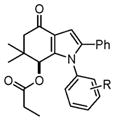 4c–4j |
4e, H (1a) | 18 | 86 |
| 6 | 4f, 4-Fluoro (1b) | 24 | 82 | |
| 7 | 4g, 4-Chloro (1c) | 20 | 84 | |
| 8 | 4h, 4-Bromo (1d) | 18 | 85 | |
| 9 | 4i, 4-Methyl (1e) | 16 | 87 | |
| 10 |
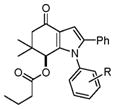 4k–4n |
4j, 4-Methoxy (1f) | 15 | 89 |
| 11 | 4k, 4-Chloro (1b) | 22 | 79 | |
| 12 | 4l, 4-Bromo (1d) | 20 | 75 | |
| 13 | 4m, 4-Methyl (1e) | 18 | 81 | |
| 14 | 4n, 4-Ethoxy (1f) | 18 | 84 | |
| 15 |
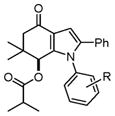 4o–4s |
4o, 4-Fluoro (1b) | 26 | 82 |
| 16 | 4p, 4-Chloro (1c) | 22 | 77 | |
| 17 | 4q, 4-Bromo (1d) | 22 | 79 | |
| 18 | 4r, 4-Methyl (1e) | 18 | 84 | |
| 19 | 4s, 4-Methoxy (1f) | 18 | 85 | |
| 20 |
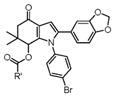 4t–4v |
4t, Methyl (3a) | 20 | 79 |
| 21 | 4u, Ethyl (3b) | 22 | 76 | |
| 22 | 4w, Propyl (3c) | 22 | 78 | |
| 23 | 4v, Isopropyl (3d) | 20 | 80 |
Reagents and conditions: 120 °C, microwave heating.
Isolated yield.
