Figure 2. General reaction scheme.
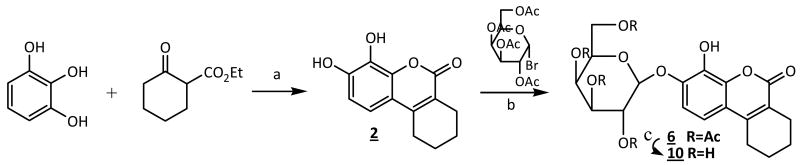
(a) pyrogallol (5 mmol), ethyl cyclohexanone-2-carboxylate (5 mmol), ZrCl4 (0.5 mmol), toluene (20 mL), 80 °C, N2, 20 min, 93%(→2); (b) 2, 3, 4, 6-tetra-O-acetyl-α-D-galactopyranosyl bromide (2.5 mmol), 2 (2.5 mmol), TBAB (0.5 mmol), CH2Cl2-H2O (60 mL), pH 8∼9, rt °C, N2, 3∼4 hr, 88%(→6); (c) 0.5M NH3-MeOH, 0°C→r.t., 24 hr, quantitative yields.
