Abstract
Objectives
To describe the frequency, indications, and outcomes following inferior vena cava (IVC) filter placement in a population-based sample of residents of the Worcester, MA, metropolitan area diagnosed with acute venous thromboembolism (VTE) in 1999, 2001, and 2003.
Methods
A retrospective chart review of inpatient and outpatient medical records was conducted. Recorded indication(s) for IVC filter placement was determined among a subset of cases from three Worcester tertiary care hospitals. Three thrombosis specialists assessed the appropriateness of IVC filter placement.
Results
Among 1547 greater Worcester residents with validated acute VTE and without a prior IVC filter, 203 (13.1%) had an IVC filter placed after acute VTE. Patients with an IVC filter placed were older, had more co-morbidities, and had higher mortality during 3-year follow-up. There was unanimous agreement by panel members that use of an IVC filter was appropriate in 51% of cases, and inappropriate in 26% of cases, with no consensus in the remaining 23%.
Conclusions
In this community-based study, IVC filters were frequently utilized in the management of patients with acute VTE. Placement was deemed to be appropriate in approximately half of the patients, but was not appropriate or debatable in the remaining cases. Given increasing use of IVC filters, prospective studies are clearly needed to better define the indications for, and efficacy of, IVC filter placement.
Introduction
Historically, insertion of an inferior vena cava (IVC) filter in patients with acute venous thromboembolism (VTE) is the last therapeutic option taken to prevent pulmonary embolism (PE). The 2001 American College of Chest Physician (ACCP) Guidelines recommended placement of an IVC filter only if there was either a contraindication to or complication of anticoagulation therapy, or if recurrent thromboembolism occurred despite adequate anticoagulation therapy.1 Insertion of a filter has never been recommended as a primary treatment of VTE.
There are risks associated with the use of IVC filters including bleeding, incorrect positioning/dislodgement, local thrombosis, and a 2-fold increase in the risk of recurrent lower extremity deep-vein thrombosis (DVT).2 The most recent 2008 ACCP guidelines for the treatment of VTE have narrowed their recommendations for filter placement even further, advocating that an IVC filter be considered only among patients with acute proximal lower extremity DVT in whom “anticoagulant therapy is not possible, because of the risk of bleeding”.3
Unfortunately, there is very little population-based data describing how frequently IVC filters are inserted in patients with acute VTE, and how often these patients have a documented failure of, or contraindication to, anticoagulant therapy. The objectives of the present study were to determine the frequency of IVC filter use in the setting of acute VTE, ascertain the indication(s) for filter placement, and determine if patients treated with an IVC filter met existing guidelines for filter placement.
Methods
The Worcester VTE study is a retrospective population-based surveillance study of VTE in Worcester, MA in 1999, 2001, and 2003.4, 5 Study years were chosen based on funding cycles, resource considerations, the desire to track events over time, and to correlate with publication of updated ACCP guidelines for the management of VTE.
Computerized printouts of all Worcester residents with healthcare encounters during calendar years 1999, 2001, and 2003 who were coded as having any of 34 ICD-9 diagnosis codes possibly consistent with VTE were obtained from each of the 12 hospitals serving the Worcester metropolitan area.4, 5 These data queries were not limited to discharge diagnoses but also encompassed all outpatient activities.
The medical records of all patients who met the geographic inclusion criteria were reviewed and validated by trained abstractors. Each case of VTE was classified as being either definite, probable, possible, or not acute/negative based on a modification of the classification schema used by Silverstein et al (see Appendix A).6 If the classification of VTE was not immediately clear using the specified criteria, the principal investigator (FAS) reviewed the medical record. For purposes of this study, patients in whom an IVC filter was placed prior to the index VTE event were excluded from further analysis.
The paper and electronic medical records of each patient’s index as well as previous hospitalizations and/or outpatient visits at participating Worcester hospitals were reviewed to determine if the index VTE event represented an incident (initial) or a recurrent case and to obtain additional information about patient co-morbidities and risk factors for VTE. Follow-up hospital record reviews to ascertain the specified outcomes of recurrent VTE and bleeding complications were conducted at a minimum of 1 year, and maximum of 3 years, following the index VTE event. State and national mortality records were reviewed on an annual basis (last reviewed in 2007) for purposes of assessing patient’s vital status; follow-up of vital status was obtained in >99% of all patients.
Data collection
Information abstracted from medical records included demographic and clinical characteristics, results of all tests for VTE, and hospital treatment and management, including date of IVC filter insertion. Medical history variables defined as “recent” were those occurring or active in the 3 months prior to the index diagnosis of VTE. Medical records for each subject at the other Worcester area hospitals were also screened in case patients sought treatment for their initial VTE event or subsequent complications at more than one area hospital.
Major bleeding was defined as any episode of bleeding requiring transfusion or hospitalization, or that was life threatening (resulted in myocardial infarction, stroke, or death). The nurse abstractors were instructed to include only those cases of bleeding that met 1 or more of these criteria as outcome events. For the 1999, 2001, and 2003 cohorts, major bleeding events were not independently reviewed by the lead investigator.
Potential recurrent VTE events were classified using criteria identical to that employed for incident cases, with the exception that a definite recurrence of DVT or PE required the documentation of thrombosis in a previously uninvolved venous or pulmonary arterial segment, respectively by diagnostic imaging (compression ultrasound, ventilation perfusion imaging, or CT scan). The lead investigator reviewed the medical record and diagnostic imaging reports of each case of potential VTE recurrence - only definite or probable recurrences were included in this study.
In the subset of patients (n = 165) seeking care at one of the three participating major tertiary care centers in the city of Worcester, indication for IVC filter insertion was ascertained by an additional careful retrospective review of the medical record. Admission, progress, and discharge notes written by the primary physician, or any consultant involved in the placement of an IVC filter, were reviewed to identify the documented indication for IVC filter use or the attending physician’s rationale for insertion of the IVC filter.
Using this abstracted data, the documented rationale for IVC filter placement was classified by the lead author (FAS) into 9 groups: a) perception of increased bleeding risk with anticoagulants, b) bleeding within 30 days prior to VTE, c) major bleeding after initiation of anticoagulants, d) minor bleeding after initiation of anticoagulants e) suspected PE occurring after initiation of anticoagulants, f) DVT occurrence/extension despite anticoagulants, g) perceived high risk for subsequent PE despite anticoagulants, h) planned interruption of anticoagulants for future surgeries or procedures, and i) other. These categories were created to simplify analysis and reporting of our observations but were not strictly defined or necessarily exclusive.
Three experts in the field of thromboembolism (FAS, RHW, SMB) independently reviewed the data pertaining to rationale/indication for filter placement for each of the patients in whom an IVC filter was placed; each of these cases was categorized as meeting or not meeting 2001 ACCP guidelines. The criteria for appropriate filter placement were: 1) documented recurrent VTE during adequate anticoagulant therapy, 2) presence of a contradiction or complication of anticoagulation in a patient with, or at high risk of, having a proximal lower extremity DVT, 3) chronic recurrent embolism with pulmonary hypertension, and 4) concurrent performance of surgical pulmonary embolectomy or pulmonary thromboendarterectomy. Agreement was measured as the proportion of cases with complete agreement for placement of an IVC filter, the proportion with complete agreement for not placing a filter, the proportion with 2 raters favoring a filter, the proportion of raters with one favoring use of a filter. The results were analyzed using the Fleiss Kappa nominal scale agreement among many raters.7
Data Analysis
The prevalence of pre-specified baseline co-morbidities in patients with VTE in whom a filter was placed was compared to patients in whom this device was not placed using chi-square tests of statistical significance for categorical variables and t-test for continuous variables. The incidence rates of our principal study outcomes among patients who received and did not receive an IVC filter were compared using Kaplan-Meier survival curves. Subjects were censored at time of event, death, or date of last medical chart documentation, whichever came first.
Results
The study sample consisted of 1,547 male and female residents of the Worcester metropolitan area with confirmed acute DVT (66%), PE (19%), or both (15%). Of these, 203 (13.1%) patients had an IVC filter placed during the index hospital stay after diagnosis of acute VTE. The median duration of follow-up was 926 days. During 1999, 2001, and 2003, 63 of 472 (13%), 75 of 547 (14%), and 65 of 528 (12%) patients had IVC filters inserted, respectively.
Characteristics of Patient Who Underwent IVC Filter Placement
Patients who received an IVC filter were more likely to be older, male, diagnosed with VTE after hospital admission for a different principal medical condition, have a medical or surgical hospitalization within the prior 3 months, require care in an intensive care unit prior to VTE diagnosis, or have other illnesses or co-morbidities present (Table 1). Patients treated with an IVC filter were also more likely to have a platelet count < 100,000 at the time of the VTE diagnosis, to have been diagnosed with a recurrent VTE, to have had a recent episode of bleeding, and to have been diagnosed with DVT together with PE (rather than isolated DVT or PE). Seven patients who underwent IVC filter placement had an isolated calf DVT.
Table 1.
Demographic and Clinical Characteristics of Patients with Venous Thromboembolism According to Placement of IVC filter*
| Variable | IVC filter placed (n = 203) |
No IVC filter (n=1344) |
P-value |
|---|---|---|---|
| Demographic Factors | |||
| Age (yrs, mean, ± SD) | 68.4 ± 16.6 | 64.3 ± 17.7 | |
| <55, % (n =446) | 19.7 | 30.2 | 0.01 |
| 55–64, % (n = 228) | 14.8 | 14.7 | |
| 65–74, % (n = 285) | 20.2 | 18.2 | |
| ≥75, % (n = 587) | 45.3 | 36.9 | |
| Male, %) (n = 697) | 52.2 | 44.0 | 0.03 |
| Medical characteristics (%) | |||
| Hospitalization history | |||
| Admitted with non-VTE diagnosis (n = 415) | 48.3 | 23.6 | <0.001 |
| Prior hospitalization < 3 mo (n = 582). | 45.3 | 36.5 | 0.04 |
| Comorbidity | |||
| Prior VTE (n = 252) | 15.3 | 16.4 | 0.67 |
| Surgery < 3 m* (n = 414) | 34.0 | 25.7 | 0.02 |
| Malignancy* (n = 447) | 36.5 | 27.8 | 0.01 |
| CNS malignancy (n = 21) | 2.5 | 1.2 | 0.18 |
| Chemotherapy < 3 mo (n = 115) | 7.9 | 7.4 | 0.80 |
| Fracture (n = 174) | 13.3 | 10.9 | 0.33 |
| Cerebrovascular accident (n = 192) | 22.7 | 10.9 | <0.001 |
| Severe infection* (n = 371) | 36.5 | 22.1 | <0.001 |
| Congestive heart failure (n = 117) | 13.3 | 6.7 | 0.002 |
| ICU discharge* (n = 242) | 34.0 | 12.9 | <0.001 |
| Intubation* (n = 233) | 30.5 | 12.7 | <0.001 |
| Other medical conditions | |||
| Prior spinal cord injury (n = 17) | 2.5 | 0.9 | 0.05 |
| Ischemic heart disease (n = 330) | 26.1 | 20.6 | 0.08 |
| Inflammatory bowel disease (n = 31) | 3.0 | 1.9 | 0.33 |
| Liver disease (n = 14) | 1.5 | 0.8 | 0.39 |
| Chronic renal insufficiency (n = 66) | 7.9 | 3.7 | 0.01 |
| Dialysis dependent (n = 19) | 3.9 | 0.8 | 0.002 |
| Platelet count < 100,000 at time of VTE diagnosis (n = 50) | 9.87 | 2.6 | <0.001 |
| Type of VTE | |||
| Isolated DVT (n = 1021) | 61.4 | 66.8 | 0.006 |
| Isolated PE (n = 292) | 14.4 | 19.6 | |
| DVT and PE (n = 232) | 24.3 | 13.6 | |
| Unprovoked (n = 377) | 7.4 | 26.9 | <0.001 |
| Provoked (n = 723) | 56.2 | 45.3 | |
| Malignancy-related (n = 447) | 36.5 | 27.8 | |
| Isolated calf DVT (n = 133) | 3.5 | 9.4 | 0.002 |
| Definite DVT (n = 1224) | 96.6 | 95.7 | 0.85 |
| Probable DVT (n = 2) | 0.0 | 0.2 | |
| Possible DVT (n = 35) | 3.4 | 4.1 | |
| Definite PE (n = 269) | 56.4 | 50.5 | 0.72 |
| Probable PE (n = 119) | 19.2 | 23.3 | |
| Possible PE (n = 135) | 24.4 | 26.2 | |
| Hospital complications (%) before IVC filter placed but after index VTE | |||
| Major bleeding (n = 38) | 12.8 | 2.8 | <0.001 |
| Recurrent VTE (n = 12) | 3.5 | 0.9 | 0.009 |
| In-hospital mortality (n = 88) | 9.9 | 5.1 | 0.01 |
| Treatments at hospital discharge† | |||
| LMWH alone (n = 105) | 6.6 | 7.3 | <0.001 |
| Warfarin alone (n = 662) | 18.6 | 49.2 | |
| LMWH and warfarin (n = 456) | 7.1 | 34.7 | |
| Neither (n = 236) | 67.8 | 8.8 |
Recent = active or occurring within 3 months of diagnosis of VTE
among hospital survivors
Treatment and Outcomes
Among the patients who were discharged from the hospital, patients with an IVC filter were much less likely to be discharged on anticoagulation therapy (low-molecular weight heparin or warfarin) (Table 1).
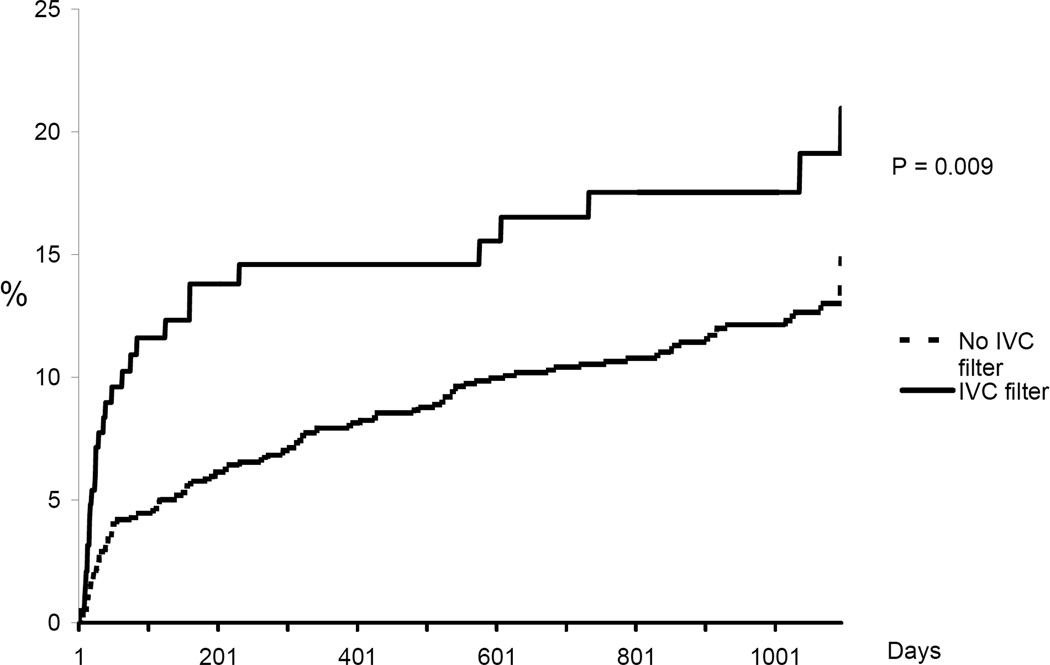
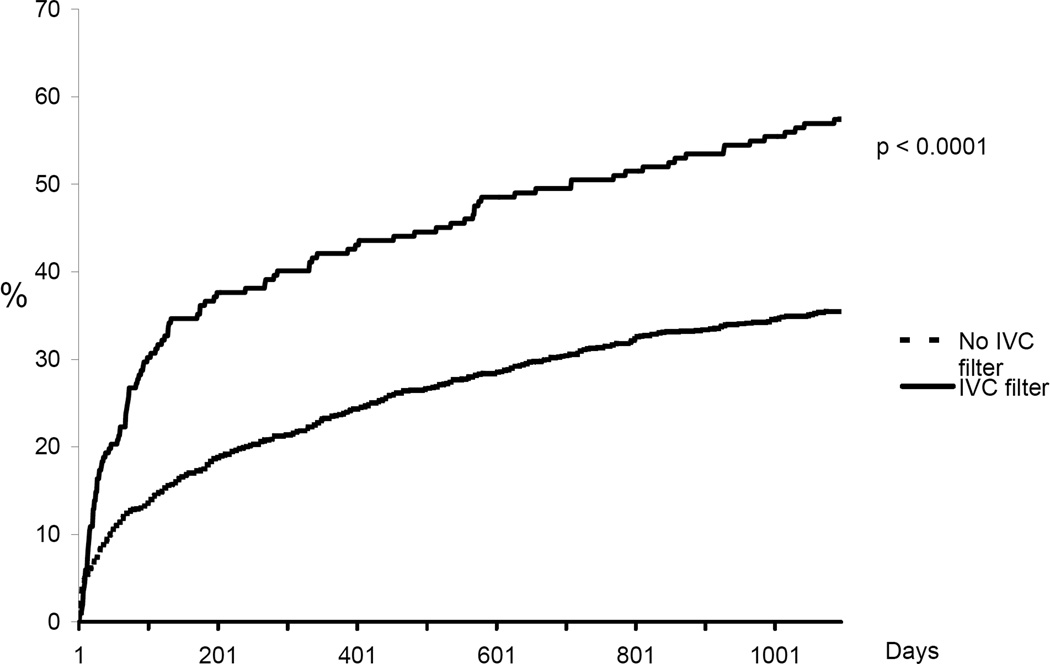
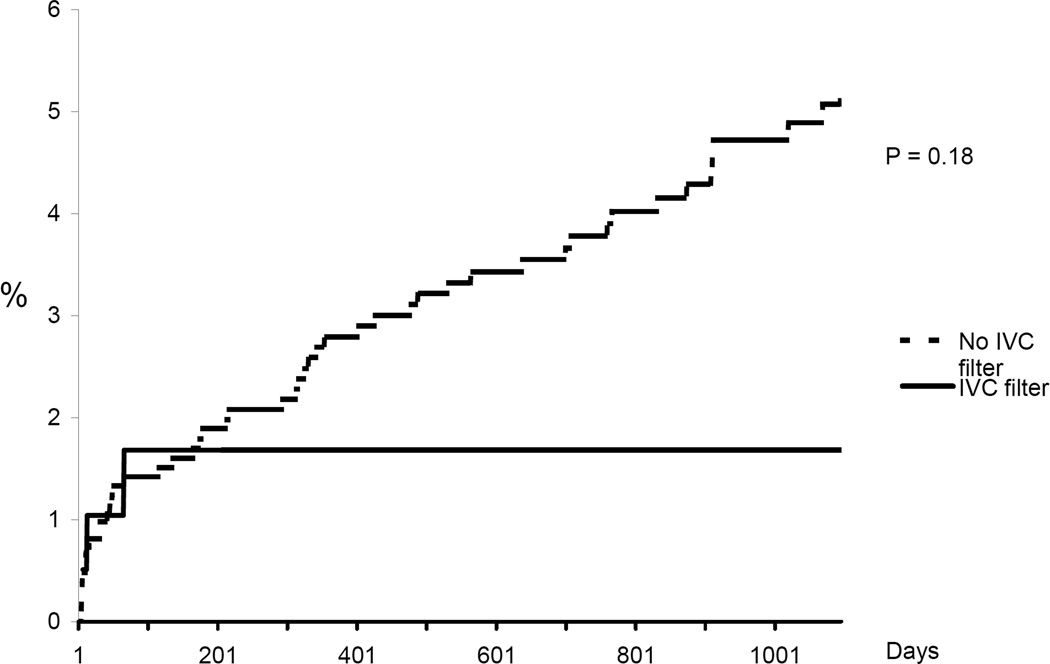
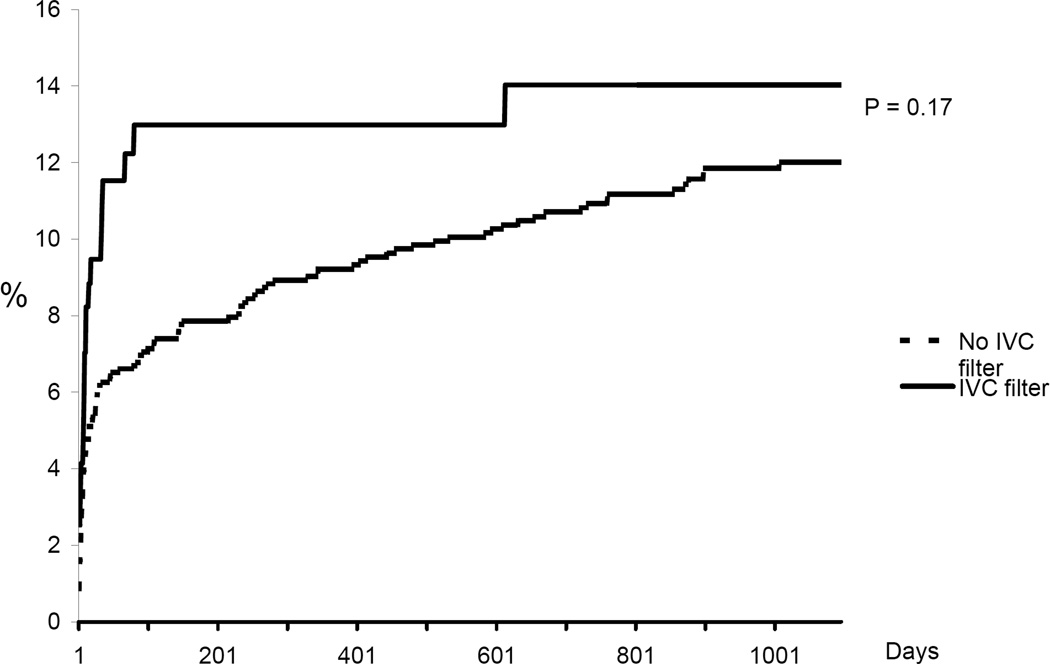
In-hospital mortality was significantly higher among patients in whom an IVC filter was placed compared to those not treated with a filter (9.9% vs. 5.1%, p =0.01). Cumulative incidence rates of recurrent DVT, new PE, major bleeding, and death are shown in Figures 1–4. The 90-day incidence of objectively confirmed PE was similar among the patients treated (1.7%) and not treated (1.4%) with an IVC filter. However, after 90 days no further PE events were diagnosed in the IVC filter treated patients whereas the cumulative incidence of PE had increased to 5.3% among the patients without a filter. Three years after the index event, the incidence of recurrent DVT was 21.0% among IVC treated patients and 14.9% among those without a filter. The incidence of major bleeding (including only bleeding after initiation of anticoagulant therapy in the non-filter group and after placement of IVC filter in the filter group) was not significantly different between the two groups. All-cause mortality was significantly higher in patients in whom an IVC filter was placed compared to patients who did not receive an IVC filter.
Figure 1.
Cumulative incidence of recurrent DVT. Kaplan Meier analysis censoring for event, death, or date of last medical record documentation.
Figure 4.
Cumulative incidence rate of all-cause mortality. Kaplan Meier analysis censoring for death or date of last medical record documentation.
Indications for IVC Filter Placement
Among the 165 patients who had an IVC filter placed in one of 3 area hospitals, the indications for filter placement were determined in 160. In 5 patients some or all of the chart information required was not available. Based on medical record review, 57 patients were deemed to be at high risk for bleeding (but had no recent bleeding), 39 had a history of recent bleeding, 26 had major bleeding after starting anticoagulation therapy, and 9 patients had minor bleeding after initiation of anticoagulation. Seven patients suffered a presumed PE after initiation of anticoagulant treatment (1 to 44 days) after the index VTE event. In one patient warfarin had been stopped for unspecified reasons for 6 days prior to a PE occurring on day 44. In two other patients, initial PTT were less than 55 seconds and PE developed on day 1 and 2, respectively. An additional 7 patients received an IVC filter after the index VTE because it represented “failure” of anticoagulant treatment [for prior VTE (n = 5), atrial fibrillation (n = 1), or stroke (n = 1)]. Of these, 2 patients had a subtherapeutic INR at the time of admission. Eight patients who were treated with full dose anticoagulant therapy also received a filter in order to prevent PE, three patients had a filter placed because of a planned procedure, and four patients had another “indication”.
Overall the 3 reviewers agreed that there was an indication for IVC filter placement in 81 of 160 patients (51%), but they also agreed unanimously that an IVC filter was not indicated in 41/160 (26%) of the patients (overall agreement 122/160 or 77%). In 20 cases, two of the reviewers felt an IVC filter was indicated but one reviewer disagreed. The Fleiss Kappa score was consistent with substantial agreement between multiple raters (K = 0.65).
Agreement and disagreement between the reviewers about whether the indication for IVC filter placement was consistent with the ACCP 2001 guidelines is shown in Table 2. Among the 39 patients with a recent history of bleeding, all 3 reviewers agreed that filter placement was appropriate in 34/39 (87%). Similarly, there was agreement that use of a filter was indicated in 23 of 26 (88%) patients who had major bleeding after starting anticoagulation therapy. Among the 57 patients who were simply judged to be at increased risk for bleeding, there was unanimous agreement that a filter was indicated in 21/57 (37%); there was also unanimous agreement that an IVC filter was not indicated in 21 of the 57 (37%) patients. In the remaining 15 patients (26%), there was disagreement among the reviewers.
Table 2.
Listed Indications for IVC filter and Panel Review of “Appropriateness of Indication”
| IVC Filter placed (n = 160) |
Panel Opinion* Indicated Not Indicated Disagree |
|||
|---|---|---|---|---|
| Perception of increased bleeding risk with anticoagulants | 57 | 21 | 21 | 15 |
| Bleeding within 30 days prior to VTE | 39 | 34 | 0 | 5 |
| Major Bleeding after initiation of anticoagulants | 26 | 23 | 1 | 2 |
| Minor Bleeding after initiation of anticoagulants | 9 | 2 | 1 | 6 |
| Presumed PE occurring after initiation of anticoagulants | 7 | 0 | 3 | 4 |
| DVT occurrence/extension despite anticoagulants | 7 | 0 | 3 | 4 |
| High risk for subsequent PE despite anticoagulants | 8 | 0 | 8 | 0 |
| Planned interruption of anticoagulants for future surgeries or procedures | 3 | 0 | 2 | 1 |
| Other | 4 | 1 | 2 | 1 |
Indicated= All 3 members agreed IVC filter placement was reasonable
Not Indicated = All 3 members felt IVC filter was not indicated
Disagree = Disagreement among members regarding appropriateness of IVC filter
Among the 14 patients who had recurrent DVT or new PE, there was agreement that a filter was not indicated in six patients whereas there was no agreement in the remaining eight patients. Among the eight patients in whom the rationale for filter placement was to prevent PE (in addition to anticoagulants), there was universal agreement that filter placement was not indicated.
Discussion
Our study suggests that approximately 1 in every 8 patients with a documented episode of VTE will receive an IVC filter in their early management. As far as we are aware, no other population-based study has reported on the frequency of IVC filter use as an acute management strategy for VTE. However, a number of studies have suggested that the utilization rates of IVC filters overall (for VTE prophylaxis and/or treatment) is growing rapidly. In a retrospective study using the National Hospital Discharge Survey database, the overall use of IVC filters was estimated to have increased from approximately 2,000 in 1979 to more than 49,000 in 1999.8 In a more recent single centre study, placement of IVC filters in patients with VTE approximately doubled between 2004 and 2007.9
Indications for IVC filter placement
While the rate of IVC filter use as an acute management strategy for VTE in our study was higher than we had anticipated, our data suggests that in the majority of patients, this treatment strategy was appropriate. Patients in whom IVC filters were placed were significantly older, were much more likely to have had recent bleeding, and had an increased prevalence of a number of co-morbidities (e.g., recent surgery or ICU stay, active malignancy, prior cerebrovascular accident) that may have predisposed them to an increased risk of serious bleeding on anticoagulation.
Given the complexity of these patients, and to gain a better understanding of specific indications for IVC filter placement, we performed an additional chart review in a subset of patients. Approximately 40% of patients suffered bleeding after their index episode of VTE or had bled in the prior 30 days. Our review panel agreed unanimously with IVC filter placement in most of these cases as this was clearly consistent with prior ACCP guidelines.1
Guidelines also indicate that IVC filter placement is appropriate in patients deemed to be at high risk for bleeding, or with a contraindication to anticoagulants, but do not define what constitutes high risk. Indeed, being at increased risk for anticoagulant-related bleeding was the listed indication in 35% of our reviewed cases. Interestingly, all of the experts agreed with filter placement in only 1/3 of these cases, unanimously disagreed with filter placement in an additional 1/3, and failed to reach a consensus in the remaining 1/3 of cases. This finding highlights the fact that guidelines that use implicit terms such as “at risk for bleeding” or “have a contraindication to anticoagulants” are subjective and interpretation of these terms may vary widely. Although the reviewers were cognizant of this, in one third of cases they unanimously agreed that a trial of anticoagulation was justified before resorting to placement of an IVC filter. Included in this group were patients who were deemed to be at high risk for falling, patients with a remote history of bleeding, and patients who underwent a major operation more than 2 weeks before being diagnosed with VTE.
Admittedly, predicting which patients will suffer major bleeding on anticoagulation following VTE remains difficult. Decision tools to better define the risk of anticoagulant associated bleeding have been developed.10–13 Whether such models can be used to help clinicians decide on whether or not anticoagulation is contraindicated (and an IVC filter is appropriate) will require further prospective studies in various at risk groups.
Prevention of PE (in addition to anticoagulation) or because of “anticoagulation failure” was the indication for IVC filter placement in 22 (14%) patients. The expert reviewers unanimously agreed that an IVC filter was not indicated in 14 of these 22 (64%) patients. Given the efficacy of available anticoagulation strategies, all reviewers felt that placement of IVC filter to “protect” a patient from PE (in addition to anticoagulation) was not an appropriate indication for use. Among the patients who received a filter because of “failure of anticoagulant treatment”, the reviewers felt that this rationale was not supported in 6 cases because anticoagulation was subtherapeutic at the time of the recurrent event (n = 5) or because the recurrent event was considered to be presumptive in 1 patient.
Efficacy and safety
Despite relatively frequent use, clinical data about the efficacy and safety of IVC filters in patients with acute VTE are sorely lacking. The relative lack of such data has led to somewhat vague guidelines regarding indications for filter use and the potential for overutilization. As far as we are aware, no clinical trials have evaluated the efficacy of IVC filters alone as an acute VTE treatment strategy. Utilization of an IVC filter as an adjunct to standard anticoagulation was compared to anticoagulation alone in the PREPIC study (Prévention du Risqué d’Embolie Pulmonaire par Interruption Cave), a randomized clinical trial of 372 patients with documented lower extremity DVT.2 At the 2-year followup, use of an IVC filter was associated with an increased risk of recurrent lower extremity DVT without any effect on survival.
In a large population-based case-control study, White and colleagues compared outcomes following acute VTE in 3,632 subjects who had a filter implanted with 64,333 subjects who did not receive an IVC filter.14 Similar to the results of the current study, patients who received an IVC filter were older and had more co-morbidities. Nevertheless, the incidence rates of recurrent PE at one year did not differ significantly between the patients who received an IVC filter as compared to those who did not. In the current study, however, we observed a trend towards a higher incidence of recurrent PE in the patients who were not treated with a filter over longer-term followup. The IVC filter treated patients had a lower cumulative incidence of PE at three years (1.7%) compared to patients who did not receive a filter (5.3%). A similar finding was also reported by the PREPIC study group after 8 years of followup.15
Any firm conclusions about the efficacy and utility of IVC filters alone as a therapy in patients who develop DVT will require a randomized trial of patients who meet agreed upon criteria for bleeding risk associated with the use of anticoagulant therapy.
Study limitations
Like any observational study, the present investigation has several limitations. Although we conducted a broad screening for all possible cases of VTE in the greater Worcester population, we cannot claim complete case ascertainment of index VTE events, episodes of VTE recurrence, or episodes of major bleeding. As in any retrospective study based on medical record review, the quality of data abstracted with respect to other medical conditions is limited by the quality of the medical documentation itself.
Another limitation of this study is that we did not analyze any patients diagnosed with acute VTE after 2003. During our study period, retrievable filters were not commonly used in the greater Worcester community - we suspect but cannot yet verify that the introduction of the retrievable IVC filter has resulted in even more liberal utilization of this treatment modality. Interestingly, in several studies rates of removal of temporary IVC filters have been quite low (5–20%) suggesting that most of these devices are in fact permanent.16, 17
Finally, it should be noted that our results may not be generalizable to other communities as it is likely that IVC filter utilization rates in a given community are likely impacted by local physician practice. This suggests the need for healthcare systems to evaluate practice within their own community.
Conclusions
The results of this observational community-based study document that an IVC filter is frequently inserted as part of an acute management strategy for acute VTE. In approximately 50% of all cases, placement of a filter appears to be appropriate and consistent with contemporary guidelines whereas in approximately one quarter of all cases, use of a filter was deemed to be inappropriate. Guidelines for IVC filter use could be improved by developing explicit criteria for contraindications to anticoagulant use and identification of patients who might benefit from the receipt of IVC filters.
Supplementary Material
Figure 2.
Cumulative incidence rate of subsequent/recurrent PE. Kaplan Meier analysis censoring for event, death, or date of last medical record documentation.
Figure 3.
Cumulate incidence rate of major bleeding. Kaplan Meier analysis censoring for event, death, or date of last medical record documentation.
Acknowledgements
This study was supported by a grant from the National Heart, Lung, and Blood Institute (R01-HL70283). Dr. Spencer also has a Career Investigator Award from the Heart and Stroke Foundation of Canada. Dr. Frederick Spencer had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Role of the Sponsor: The sponsors had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosures: None to report
Reference List
- 1.Hyers TM, Agnelli G, Hull RD, et al. Antithrombotic therapy for venous thromboembolic disease. Chest. 2001 Jan;119(1 Suppl):176S–193S. doi: 10.1378/chest.119.1_suppl.176s. [DOI] [PubMed] [Google Scholar]
- 2.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. 1998 Feb 12;338(7):409–415. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 3.Kearon C, Kahn SR, Agnelli G, Goldhaber S, Raskob GE, Comerota AJ. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008 Jun;133(6 Suppl):454S–545S. doi: 10.1378/chest.08-0658. [DOI] [PubMed] [Google Scholar]
- 4.Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006 Jul;21(7):722–727. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer FA, Gore JM, Lessard D, Douketis JD, Emery C, Goldberg RJ. Patient outcomes after deep vein thrombosis and pulmonary embolism: the Worcester Venous Thromboembolism Study. Arch Intern Med. 2008 Feb 25;168(4):425–430. doi: 10.1001/archinternmed.2007.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ., III Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998 Mar 23;158(6):585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 7.Fleiss JL. Measuring nominal scale agreement among many raters. Psychological Bulletin. 1971;76:378–382. Ref Type: Generic. [Google Scholar]
- 8.Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004 Jul 26;164(14):1541–1545. doi: 10.1001/archinte.164.14.1541. [DOI] [PubMed] [Google Scholar]
- 9.Singh P, Lai HM, Lerner RG, Chugh T, Aronow WS. Guidelines and the use of inferior vena cava filters: a review of an institutional experience. J Thromb Haemost. 2009 Jan;7(1):65–71. doi: 10.1111/j.1538-7836.2008.03217.x. [DOI] [PubMed] [Google Scholar]
- 10.Gage BF, Yan Y, Milligan PE, et al. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF) Am Heart J. 2006 Mar;151(3):713–719. doi: 10.1016/j.ahj.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med. 1998 Aug;105(2):91–99. doi: 10.1016/s0002-9343(98)00198-3. [DOI] [PubMed] [Google Scholar]
- 12.Kuijer PM, Hutten BA, Prins MH, Buller HR. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Arch Intern Med. 1999 March 8;159(5):457–460. doi: 10.1001/archinte.159.5.457. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-Gimenez N, Suarez C, Gonzalez R, et al. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thromb Haemost. 2008 Jul;100(1):26–31. doi: 10.1160/TH08-03-0193. [DOI] [PubMed] [Google Scholar]
- 14.White RH, Zhou H, Kim J, Romano PS. A population-based study of the effectiveness of inferior vena cava filter use among patients with venous thromboembolism. Arch Intern Med. 2000 Jul 10;160(13):2033–2041. doi: 10.1001/archinte.160.13.2033. [DOI] [PubMed] [Google Scholar]
- 15.Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study 1. Circulation. 2005 Jul 19;112(3):416–422. doi: 10.1161/CIRCULATIONAHA.104.512834. [DOI] [PubMed] [Google Scholar]
- 16.Singh P, Lai HM, Lerner RG, Chugh T, Aronow WS. Guidelines and the use of inferior vena cava filters: a review of an institutional experience. J Thromb Haemost. 2009 Jan;7(1):65–71. doi: 10.1111/j.1538-7836.2008.03217.x. [DOI] [PubMed] [Google Scholar]
- 17.Karmy-Jones R, Jurkovich GJ, Velmahos GC, et al. Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study. J Trauma. 2007 Jan;62(1):17–24. doi: 10.1097/TA.0b013e31802dd72a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.