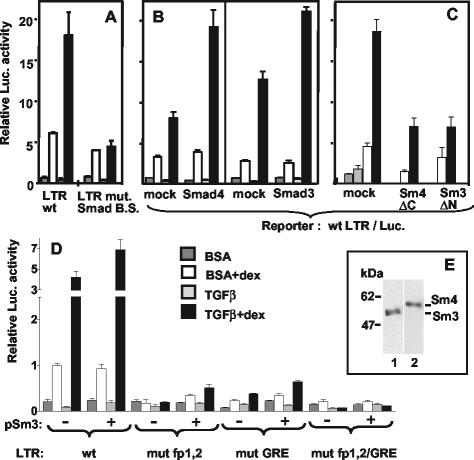
FIG. 3.
Smad proteins and a putative Smad-binding site in the MMTV LTR between fp1 and fp2 play a role in TGF-β superinduction. Transient transfection and treatment of M12 cells were as described for Fig. 1B. The internal control was pRL-SV40Δ enhancer (50 ng in panels A, B, and D and 25 ng in panel C). (A) pGL3-Basic (3 μg) with a wild-type LTR (LTR wt) or an LTR with an 8-bp mutation between fp1 and fp2 (LTR mut. Smad B.S. [see Fig. 1A]) were transfected into M12 cells grown to high density (see the text). The result is representative of two independent experiments with duplicate samples. (B) Three micrograms of pGL3-basic/wt-LTR was cotransfected with 3 μg of either empty pCI vector (mock), pCMV5-DPC4-HA expressing the Smad4 protein or pCI-Smad3F. (C) One hundred nanograms of pGL3-basic/wt-LTR were cotransfected with 8 μg of either empty pCI vector (mock), pCMV/DPC4(1-514) (Sm4 ΔC), or pCI-Smad3F ΔN (Sm3 ΔN). (D) pGL3-basic (3 μg) with a wild-type LTR (wt), or an LTR mutated in fp1 and fp2 (mut fp1,2; see Fig. 1A), or in GRE (mut GRE = LS −175/−166 [10]), or in both GABP and GR binding sites (mut fp1,2/GRE) was cotransfected with 6 μg of either pCI (−) or pCI-Smad3F (+). Error bars indicate the standard deviations. (E) Western immunoblot of a whole-cell lysate from M12 cells treated with TGF-β plus Dex. Replicated slots were blotted and assayed with antibodies to Smad2/3 (lane 1) or Smad4 (lane 2). The position of molecular size markers is indicated on the left in kilodaltons.