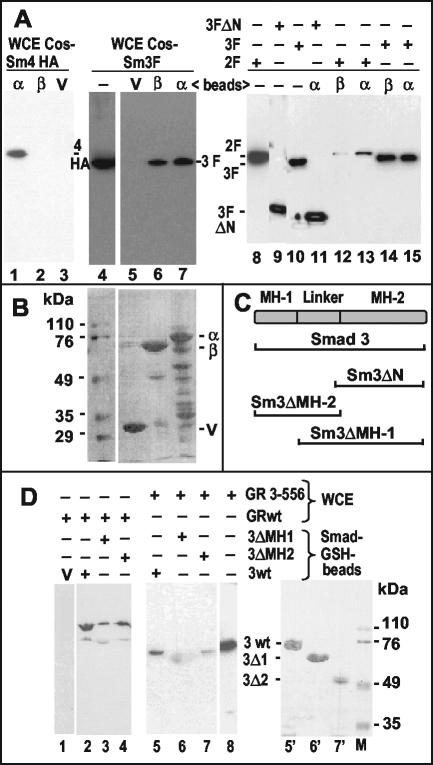
FIG. 6.
Association of Smads with GABP and GR in vitro. (A) GST pull-down assays were performed as described in Materials and Methods. GST-GABPα (α), GST-GABPβ (β), or GST vector alone (V) bound to glutathione-Sepharose beads were mixed with whole-cell lysates (WCE) of transfected COS cells expressing HA-Smad4 (lanes 1 to 3), Flag-Smad2 (lanes 12 and 13), or Flag-Smad3 (full-length in lanes 5 to 7 and lanes 14 and 15 or N terminally deleted in lane 11). The eluted complexes were analyzed by Western immunoblot with antibodies directed to the respective epitope tags. Lanes without added beads (−) contain ca. 5% of the whole-cell lysate inputs as a control. (B) Ponceau-S-stained membrane corresponding to lanes 5 to 7 of panel A showing the GST proteins. Numbers on the left identify the molecular mass markers on the same gel. (C) Scheme of the Smad3 protein and its domains. Horizontal brackets below denote the fractions of the protein present in the mutants used. (D) Beads carrying GST fusions with the indicated parts of Smad3 (see scheme in panel C) or the GST vector (V) were mixed with whole-cell lysates (WCE) of transfected COS cells expressing full-length GR (GRwt) or an N-terminal fragment (GR 3-556; see scheme Fig. 4C). The eluted complexes were analyzed by Western immunoblot with antibodies against GR that recognize its N-terminal domain. The diffuse spot in lane 6 is due to nonspecific sticking of the secondary antibody to GST-Smad3ΔMH1 (see lane 6′). Lane 8 shows the input lysate (∼5%). Lanes 5′ to 7′, Ponceau-S-stained membrane corresponding to lanes 5 to 7; lane M, molecular size markers (numbers on the right in kilodaltons).