Abstract
Background:
Titanium dioxide exists in three different crystal lattices, anatase, rutile, and brookite. Anatase coating releases, under ultraviolet (UV) irradiation, free radicals such as ·OH, O2-, HO2-, and H2O2. This potent oxidizing power characteristically results in the lysis of bacteria and other organic substances. The purpose of this study was to evaluate the bone response to implants made of titanium alloy or coated with a new combination of anatase and Bactercline® product.
Materials and Methods:
In the period between July 2009 and June 2010, 26 patients (10 females and 16 males; median age 51 ± 11 years, min. 27 years, max. 72 years) were operated and 62 implants were inserted. Lost fixtures and peri-implant bone resorption were considered as predictors of clinical outcomes. Pearson χ2-test was used. Prosthesis and implant failures, any complications after loading, and peri-implant marginal bone-level changes were assessed by a masked assessor. All patients were followed up to 1 year after loading.
Results:
No implant was lost. Average bone resorption around implant was 0.33 mm (both for 25 standard and 37 Bactercline-coated implants), and thus no statistical difference was detected.
Conclusion:
These results shown that no adverse effects on osseo-integration were present.
Keywords: Anatase, implant, nanocoating, tooth replacement
INTRODUCTION
Titanium biocompatibility is strongly related to its surface oxide layer properties, mainly its structure, morphology, and composition.[1,2,3] Several studies have been conducted in order to evaluate its biocompatibility and metal ion release. Titanium dioxide exists in three different crystal lattices, anatase, rutile, and brookite.[4] Normally, a stochastic distribution of two titanium oxides (rutile and anatase) is present on the surface of the titanium, and this is responsible for the properties of the material.[5] Anatase has been shown in in vitro studies to be able to absorb more OH– and PO4 (3–) than rutile, in body fluids, which favors depositing a bone-like apatite formation.[1,6,7]
A homogeneous anatase coating can be produced around the implant surface with different systems.[6] The anatase coating releases, under ultraviolet (UV) irradiation, free radicals such as •OH, O2-, HO2-, and H2O2. This potent oxidizing power characteristically results in the lysis of bacteria and other organic substances.[8,9,10,11] Several reports have been published on the bactericidal properties of TiO2 against organisms such as Escherichia coli.[12,13] The exact killing mechanism has not been well elucidated.[9,12] This bactericidal action could be due to the elimination of the protection of the cell wall of the bacteria, and then to an increase in the cell permeability determining a loss of intracellular contents, leading to the death of the cells.[9,12]
The aim of this controlled clinical trial was to evaluate the bone response to implants made of titanium alloy or coated with a new combination of anatase and Bactercline® product. The present report represents the follow-up up to 1 year after loading of a previous article.[14] It focuses on the peri-implant bone-level changes from implant placement.
MATERIALS AND METHODS
Any patients with partial edentulism having a residual bone height greater than 11 mm and a thickness of at least of 5 mm to allow placement at least of two implants, who was 18 or older and able to sign an informed consent form, was eligible for inclusion in this trial. Patients were not included in the study if any of the following exclusion criteria was present: (1) general contraindication to implant surgery; (2) subjected to irradiation, chemotherapy, or immunosuppressive therapy over the past 5 years; (3) poor oral hygiene and motivation; (4) uncontrolled diabetes; (5) pregnant and lactating; (6) substance abusers; (7) smoking more than 15 cigarettes per day; (8) psychiatric problem or unrealistic expectation; (9) acute infection in the area intended for implant placement; (10) positive to human immunodeficiency virus (HIV) and hepatitis B and C; (11) affected by autoimmune diseases such as arthritis rheumatoid, systemic lupus erythematosus, sclerodermia, Sjögren syndrome, and dermatomyositis/polymyositis; (12) treated or under treatment with intra-venous amino-bisphosphonates; (13) subjected previously to reconstructive procedures of the jaws; and (14) under chronic treatment with steroid and non-steroidal anti-inflammatory drugs.
This study was approved by the Ethical Committee of University of Chieti-Pescara, Chieti, Italy.
Patients
In the period between July 2009 and June 2010, 26 patients (10 females and 16 males; median age 51 ± 11 years, min. 27 years, max. 72 years) were operated and 62 implants were inserted.
Data collection
Before surgery and in the follow-up period, radiographic examinations were done with the use of an intra-oral and orthopantomograph. In addition, the following parameters were considered: Absence of persisting pain or dysesthesia, absence of peri-implant infection with suppuration, absence of mobility, and absence of persisting peri-implant bone resorption around implants. Not only lost implant but also a bone resorption greater than 1.5 mm during the first year of loading and an additional 0.2 mm for the subsequent year were evaluated. Additional details are available in a previous report.[14]
Preparation of anatase-Bactercline® coatings
A detailed description of Bactercline was reported previously.[14] Additional information is available in International Patent Application (1) WO 2007/122651—Functional Nanomaterials with Antibacterial and Antiviral Activity. 01.11.2007 B22F 1/02 PCT/IT2006/000280 and (2) WO 2008/020460—Nanomaterial Coatings for Osteointegrated Biomedical Prostheses. 21.02.2008 A61L 27/30 PCT/IT2006/000450.
Bactercline is a new disinfecting formulation, which has passed the examination of the Italian National Institute of Health, Rome, Italy, and is now classified as Presidio Medico Chirurgico N. 19258. The product was applied on the implant surfaces by dip coating after deposition of an anatase layer prepared according to the procedure, which follows.
Implants
Sixty-two implants (Dental Tech, Misinto, Italy) were inserted. Among them there were two different surfaces: 25 with an acid-etched and sandblasted surface (Control) and 37 with an acid-etched and sandblasted surface coated with an anatase (Bactercline solution, Test). Each patient received at least one Test and one Control implant placed close each other in the same surgical procedure.
Surgical and prosthetic technique
All patients underwent the same surgical protocol as described previously.[14] The choice of implant diameter and length was left to the surgeon according to the anatomical limitations. Implants were placed with the neck flush with the surrounding bone and were submerged for a healing period of 4 months. Four months later the implants were exposed, abutments were placed, and screw-retained, implant-supported acrylic resin temporary fixed crowns were delivered. Definitive metal—ceramic screw-retained prostheses or those cemented with provisional cement were inserted after an additional 4 months. Patients were enrolled in an oral hygiene program with recall visits every 3 months for the entire duration of the study.
Intra-oral radiographs were made with the paralleling technique at implant placement and 1 year after loading [Figures 1 and 2]. In the case where the bone levels around the studied implants were hidden or difficult to read, a second radiograph was made.
Figure 1.
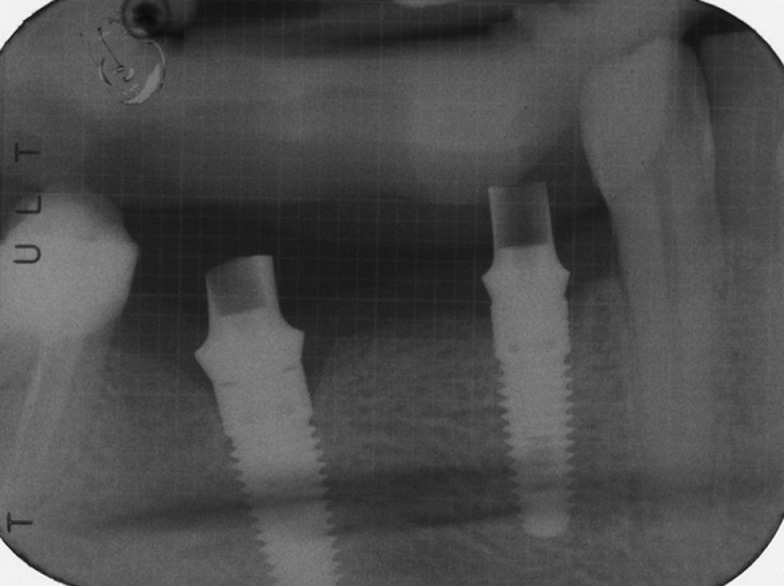
Intra-oral Rx showing implant with abutments
Figure 2.
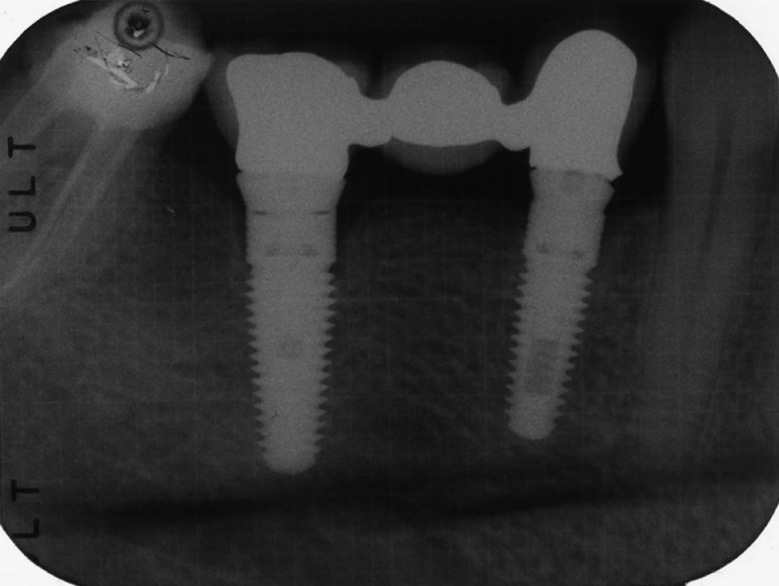
The 12-month post-loading control
All surgical intervention was performed by two experienced operators, whereas prosthesis was fabricated by different dentists. This study tested the null hypothesis that there were no differences between Test and Control implants against the alternative hypothesis of a difference. The outcome measures were:
Prosthesis failures: Planned prosthesis that could not be placed due to implant failure and loss of the prosthesis secondary to implant failure.
Implant failure: Implant mobility and removal of stable implant dictated by progressive marginal bone loss or infection.
Peri-implant marginal bone evaluated on intra-oral radiographs taken with paralleling technique. A detailed description of the technique has been reported previously.[15] Briefly, peri-apical X-rays were taken at implant placement and 1 year after loading. Radiographs were scanned, digitalized, and stored on a personal computer. Peri-implant marginal bone levels were measured by using a software calibrated for each single image using the known implant length. Mesial and distal bone crestal level adjacent to each implant were measured to the nearest 0.1 mm. Reference points for the linear measurements were the most coronal margin of the implant collar point of the bone-to-implant contact and implant tip.
Statistical analysis
Pearson χ2-test was used to investigate differences among the two groups of implants.
RESULTS
Twenty-six patients were considered eligible and were consecutively enrolled into the clinical trial. All patients were treated according the allocated interventions; no drop-out, exclusion, or deviation from the protocol occurred up to 1 year after loading, and the data of all patients were evaluated in the statistical analysis. Patients were recruited and treated with implant insertion in the period between July and June 2010, whereas all provisional restoration (i.e., loading) was delivered with December 2010.
The mean patient age at the time of surgical procedure was 51 ± 11 years (range 27-72) and there were 10 females and 16 males. In total 62 implants were inserted. Implant diameter was 3.75 and 4.50 mm in 38 and 24 fixtures, respectively. Length was 10, 11.5, and 13 mm in 25, 12, and 25 cases, respectively. Thirty-four and 28 implants were placed in the mandible and maxilla, respectively. Fixture replaced 5 incisors, 4 cuspids, 26 premolars, and 27 molars. Twenty-five implants were standard whereas 37 were Bactercline-coated.
No implant was lost. Average bone resorption around implant was 0.3 mm (both for standard and Bactercline-coated implants), and no statistical difference was detected [Tables 1 and 2].
Table 1.
Cross-tabulation showing bone resorption around implants using a cut-off equal to 1.5 mm, which is the international standard
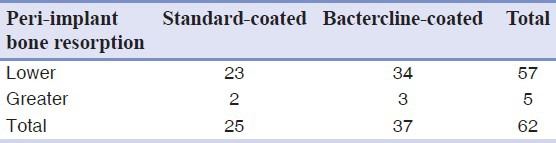
Table 2.
Cross-tabulation showing bone resorption around implants using a cut-off equal to 0.3 mm, which was the mean bone resorption
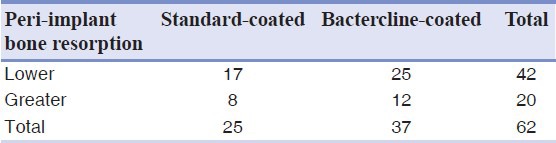
DISCUSSION
Prostheses are characterized by macro-, mini-, micro-, and nano-design. Macro-design is the shape of the prosthesis: includes the cylindrical or root form of dental implants. Mini-design is the dimension of the threads or the shape of the neck of the dental fixture. Dimension ranges from 1 to 0.1 mm. Micro-design is the shape of implant surface, as exemplified by the “grooves and holes” resulting from surface treatments such as machination, acid-etching, and sand-blasting. These treatments determine the roughness of the surface and the “holes” have a cellular dimension. Nano-design is determined by the molecular composition of the surfaces, such as those composed of hydroxypatite, zirconium, and titanium. Usually, mechanical proprieties are related to macro- and mini-design, whereas biological proprieties are related to micro- and nano-design. Mechanical proprieties are responsible for primary implant stability, whereas biological proprieties are relevant in the osseo-integration process.
When exposed to air or liquids, titanium produces a layer of oxide that reduces its reactivity, and this oxide layer interacts with the tissues.[16]
The anatase form of TiO2 is one of the most common crystalline forms of TiO2, and is normally produced by oxidation of titanium via thermal oxidation or anodization.[17] This crystalline form shows photocatalytic activity when irradiated with UV-A light.[17] This photocatalytic activity produces decomposition of several organic compounds.[17] Recently, in a study in our laboratory, it has been demonstrated that coating of healing screws with a derivate of anatase (i.e., Bactercline) produced a lower quantity of bacteria on the surface of these screws.[18] There could be some concern on the use of this Bactercline coating on dental implants due to a possible interference with the osteoblastic activity.
A preliminary report[14] demonstrated that no adverse effect on osseo-integration was present, and no statistically significant differences between Test and Control implants was detected after a mean follow-up of 7 months. As shown in the work by Lauritano and co-workers,[19,20,21] bacteria can determinate bone re-absorption around dental implant caused by peri-implantitis.
CONCLUSIONS
Use of this coating at the implant body could have positive effects in cases of peri-implant crestal bone resorption during peri-implantitis, when a coating that could help in decreasing the bacterial charge could be helpful in the treatment of peri-implant infection.
Here, additional strength is given to previous results since results remain stable over a longer observation period.
ACKNOWLEDGEMENTS
This work was supported by the University of Ferrara (F.C.), Ferrara, Italy and by PRIN 2008 (20089MANHH_004).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sollazzo V, Palmieri A, Pezzetti F, Scarano A, Martinelli M, Scapoli L, et al. Genetic effect of anatase on osteoblast-like cells. J Biomed Mater Res B Appl Biomater. 2008;85:29–36. doi: 10.1002/jbm.b.30912. [DOI] [PubMed] [Google Scholar]
- 2.Joseph LA, Israel OK, Edet EJ. Comparative evaluation of metal ions release from titanium and Ti–6Al–7Nb into bio-fluids. Dent Res J. 2009;6:7–11. [PMC free article] [PubMed] [Google Scholar]
- 3.Fathi MH, Mortazavi V. Tantalum, niobium and titanium coatings for biocompa improvement of dental implants. Dent Res J. 2007;4:74–82. [Google Scholar]
- 4.Tsyganov I, Maitz MF, Wieser E, Prokert F, Richter E, Rogozin A. Structure and properties of titanium oxide layers prepared by metal plasma immersion ion implantation and deposition. Surf Coatings Technol. 2003;174-5:591–6. [Google Scholar]
- 5.Li LH, Kong YM, Kim HW, Kim YW, Kim HE, Heo SJ, et al. Improved biological performance of Ti implants due to surface modification by micro-arc oxidation. Biomaterials. 2004;25:2867–75. doi: 10.1016/j.biomaterials.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 6.Palmieri A, Brunelli G, Guerzoni L, Lo Muzio L, Scarano A, Rubini C, et al. Comparison between titanium and anatase miRNAs regulation. Nanomedicine. 2007;3:138–43. doi: 10.1016/j.nano.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Palmieri A, Pezzetti F, Brunelli G, Arlotti M, Lo Muzio L, Scarano A, et al. Anatase nanosurface regulates microRNAs. J Craniofac Surg. 2008;19:328–33. doi: 10.1097/SCS.0b013e3181534ab3. [DOI] [PubMed] [Google Scholar]
- 8.Cho M, Chung H, Choi W, Yoon J. Different inactivation behaviors of MS-2 phage and Escherichia coli in TiO 2 photocatalytic disinfection. Appl Environ Microbiol. 2005;71:270–5. doi: 10.1128/AEM.71.1.270-275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiraish K, Koseki H, Tsurumoto T, Baba K, Naito M, Nakayama K, et al. Antibacterial metal implant with a TiO 2 -conferred photocatalytic bactericidal effect against Staphylococcus aureus. Surf Interface Anal. 2008;41:17–21. [Google Scholar]
- 10.Marciano FR, Lima-Oliveira DA, Da-Silva NS, Diniz AV, Corat EJ, Trava-Airoldi VJ. Antibacterial activity of DLC films containing TiO 2 nanoparticles. J Colloid Interface Sci. 2009;340:87–92. doi: 10.1016/j.jcis.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 11.Asahara T, Koseki H, Tsurumoto T, Shiraishi K, Shindo H, Baba K, et al. The bactericidal efficacy of a photocatalytic TiO(2) particle mixture with oxidizer against Staphylococcus aureus. Jpn J Infect Dis. 2009;62:378–80. [PubMed] [Google Scholar]
- 12.Maness PC, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. Bactericidal activity of photocatalytic TiO(2) reaction: Toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65:4094–8. doi: 10.1128/aem.65.9.4094-4098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibanez JA, Litter MI, Pizarro RA. Photocatalytic bactericidal effect of TiO2 on Enterobacter cloacae comparative study with other Gram (-) bacteria. J Photochem Photobiol A. 2003;157:81–5. [Google Scholar]
- 14.Brunelli G, Carinci F, Grecchi F, Avantaggiato A, Piattelli A, Scarano A, et al. Bactercline ® coated implants inserted in maxilla grafted with bone from living donor: A case report. Eur J Inflamm. 2011;9:61–5. [Google Scholar]
- 15.Grecchi F, Pagliani L, Mancini GE, Zollino I, Carinci F. Implant treatment in grafted and native bone in patients affected by ectodermal dysplasia. J Craniofac Surg. 2010;21:1776–80. doi: 10.1097/SCS.0b013e3181f40378. [DOI] [PubMed] [Google Scholar]
- 16.Olmedo DG, Tasat DR, Evelson P, Guglielmotti MB, Cabrini RL. Biological response of tissues with macrophagic activity to titanium dioxide. J Biomed Mater Res A. 2008;84:1087–93. doi: 10.1002/jbm.a.31514. [DOI] [PubMed] [Google Scholar]
- 17.Sawase T, Jimbo R, Wennerberg A, Suketa N, Tanaka Y, Atsuta M. A novel characteristic of porous titanium oxide implants. Clin Oral Implants Res. 2007;18:680–5. doi: 10.1111/j.1600-0501.2007.01404.x. [DOI] [PubMed] [Google Scholar]
- 18.Scarano A, Degidi M, Iezzi G, Pecora G, Piattelli M, Orsini G, et al. Maxillary sinus augmentation with different biomaterials: A comparative histologic and histomorphometric study in man. Implant Dent. 2006;15:197–207. doi: 10.1097/01.id.0000220120.54308.f3. [DOI] [PubMed] [Google Scholar]
- 19.Brunelli G, Carinci F, Zollino I, Candotto V, Scarano A, Lauritano D. SEM evaluation of 10 infected implants retrieved from man. Eur J Inflamm. 2012;10:7–12. [Google Scholar]
- 20.Brunelli G, Carinci F, Zollino I, Candotto V, Scarano A, Lauritano D. Peri-Implantitis: A case report and literature review. Eur J Inflamm. 2012;10:1–6. [Google Scholar]
- 21.Scarano A, Murmura G, Carinci F, Lauritano D. Immediately loaded small diameter dental implants: Evaluation of retention, stability and comfort for the edentulous patient. Eur J Inflamm. 2012;10:19–24. [Google Scholar]


