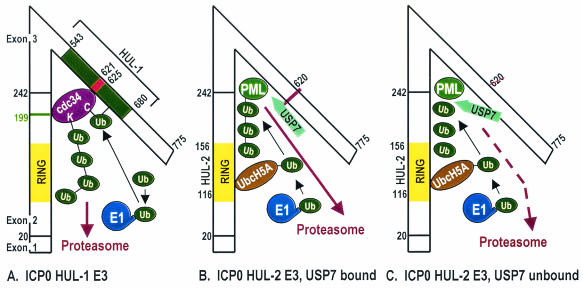
FIG. 1.
Models showing interactions of ICP0 with the ubiquitin-proteasome system. (A) HUL-1 E3 domain. ICP0 HUL-1 E3 activity, mapping in sequences encoded in exon 3, promotes the autoubiquitylation and degradation of the E2 enzyme cdc34 (62-64, 152). The model depicted is described in the text. The figure is adapted from reference 62. (B) RING finger (HUL-2) E3 domain mapping to sequences encoded by exon 2. ICP0 RING finger E3 activity promotes the ubiquitylation and degradation of PML and other cellular proteins (13, 45, 46, 95, 120, 122). While the ICP0 RING finger has E3 ligase activity in in vitro reactions containing either UbcH5a or UbcH6 E2 ubiquitin-conjugating enzymes (14, 64), the degradation of PML and Sp100 appears to be mediated by UbcH5a (60). The E2 enzyme(s) involved in the degradation of the other known substrates of the ICP0 RING finger has not been identified. Available data indicate that ICP0 binds USP7 and sequesters it from deubiquitylating substrates ubiquitylated by ICP0. (C) Model of HUL-2 E3 in cells infected with a mutant incapable of binding USP7. The model shows that unbound USP7 would be available to deubiquitylate newly ubiquitylated ICP0 substrates, decreasing the efficiency of their degradation. This model is consistent with data showing that mutants unable to bind USP7 have a reduced capacity to degrade PML and Sp100 (42; Hagglund and Roizman, unpublished).