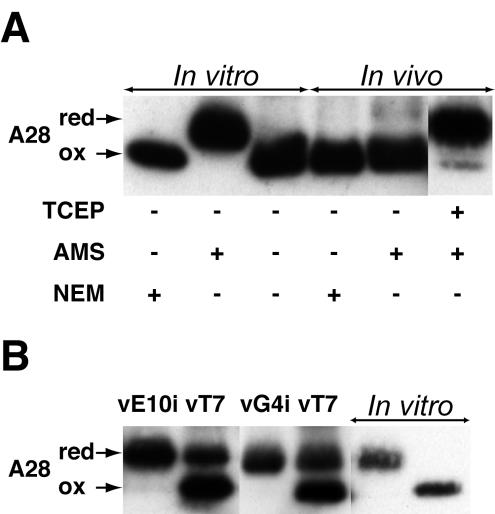
FIG. 3.
Formation of disulfide bonds in A28-HA. (A) A28-HA contains two intramolecular disulfide bonds. In vitro, A28-HA synthesized in a coupled transcription-translation recticulocyte lysate system was captured with anti-HA antibody, and aliquots were disrupted in SDS-PAGE loading buffer containing (+) NEM or AMS or no alkylating agent (−), as indicated. In vivo, cells were infected with vA28-HA, collected 24 h after infection, and disrupted in SDS-PAGE loading buffer containing NEM, AMS, or Tris-(2-carboxyethyl) phosphine (TCEP), followed by AMS. Proteins were resolved by SDS-PAGE; A28L-HA was detected by Western blotting with anti-HA antibody. A28, A28-HA; red, reduced A28-HA; ox, oxidized A28-HA. (B) Expression of E10 and G4 is required to form disulfide bonds in A28. Cells were infected with either vE10i or vG4i without IPTG or with vT7lacOI (vT7) as a control and transfected with plasmid expressing A28-HA under the control of the strong late vaccinia virus promoter P11. Cells were collected 20 h after infection. Proteins from infected and transfected cells were allowed to react with AMS and were analyzed as for panel A. A28-HA made in vitro was treated with AMS (next to last lane on right) or NEM (last lane on right) to provide mobility markers.