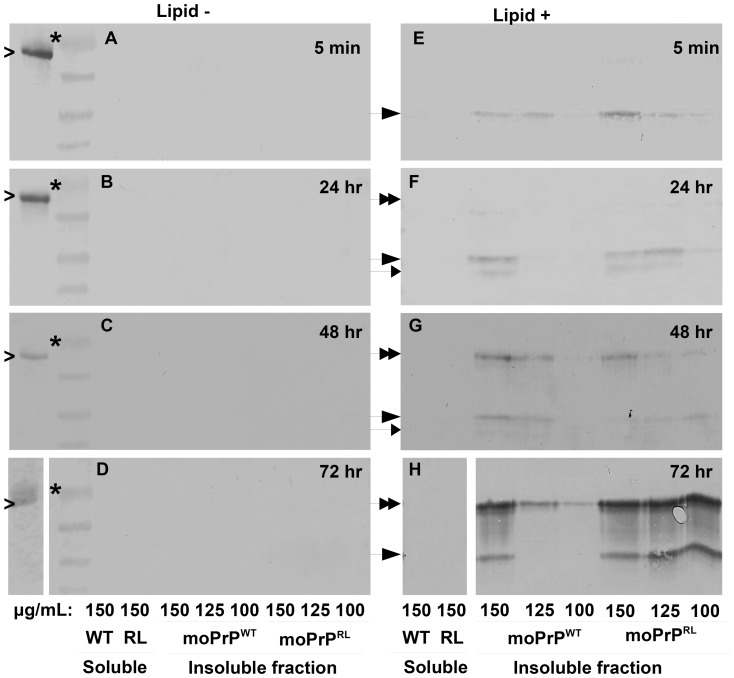
Figure 1. moPrPRL converts to a PK resistant isoform at lower concentrations than moPrPWT.
moPrPWT and moPrPRL at three different concentrations were incubated in the presence (E–H) or absence (A–D) of POPG lipid vesicles. Samples were removed for proteolytic digestion by proteinase K (PK) followed by ultra-centrifugation and Western blot analysis at four different time points. Generation of protease resistant, insoluble PrP was compared over time between moPrPWT and moPrPRL. In POPG vesicle containing samples, generation of material that is both insoluble and resistant to PK digestion was detectible after only five minutes (E) as a 15 kDa PrP band (denoted by large arrow). A smaller molecular weight band of approximately 14.5 kDa (denoted by small arrow) was detectible by 24 hours (F). Conversion to a PK resistant, insoluble form at a concentration of 100 µg/mL is observed for only moPrPRL, consistently at all time periods analyzed (E–H, lanes 3 vs 6). Over time, PK treated, ultracentrifuged samples accumulated a 24 kDa band (denoted by double arrow) (E–H). This band may represent undigested rPrP or a dimer of smaller molecular weight, PK cleaved fragments. Generation of aggregated, PK resistant material in the absence of lipids was not detected for either moPrPWT or moPrPRL (A–D). PK resistant material was also undetectable in the soluble fraction (A–H). Data shown is representative of results from four independent experiments.