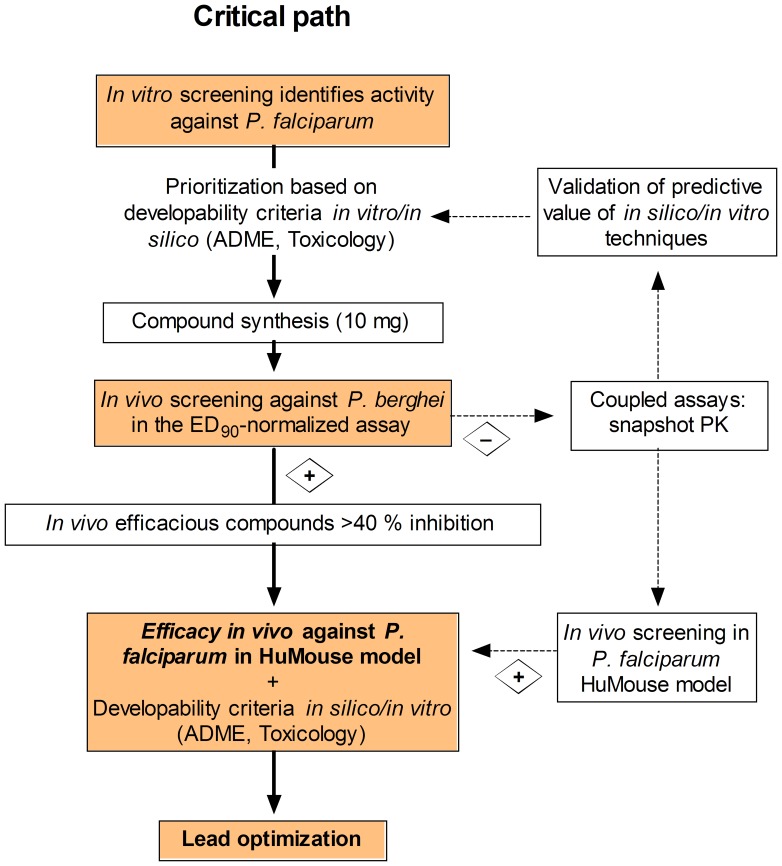
Figure 7. A new paradigm for the critical path of compound progression in antimalarial drug discovery.
Compounds with activity in in vitro phenotypic assays are rigorously scored according to available in silico/in vitro absorption, distribution, metabolism and toxicity (ADMET) predictive techniques and theoretical chemical properties. Small amounts of compound (10 mg) are synthesized in order of priority for in vivo screening in the Plasmodium berghei ED90-normalized assay. Non-efficacious compounds showing significant exposure could be rescued for re-testing in the P. falciparum humanized mouse (HuMouse) model in order to discard the risk of species selectivity. Efficacious compounds are evaluated in vitro for drug metabolism/pharmacokinetics (DMPK), toxicity, and anti-parasitic activity for identification of development risks. The lead optimization program commences with full profiling of the lead compound: in vitro cytotoxicity and preliminary genotoxicity, in vivo DMPK (bioavailability and clearance in rodents), in vitro generation of resistance and killing rate activity, and in vivo dose–response efficacy in the P. falciparum HuMouse model. Further enrichment of the outcome of the P. berghei in vivo screening can be obtained by coupling high-content secondary endpoints to the screening, such as snapshot pharmacokinetic sampling, to provide valuable information for validation and refinement of ADMET predictive tools.