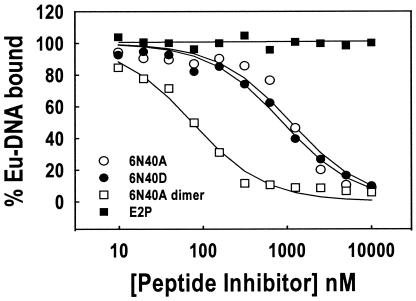
FIG. 1.
Comparison of synthetic peptides by TRF. IC50 results are reported in Table 1. One microgram of anti-E2 monoclonal antibody 93.2a12 (anti-E2 antibody generated at GlaxoSmithKline) was added to each well in a 96-well plate and incubated at 4°C overnight. Wells were blocked with 1% milk in Tris-buffered saline and then washed with TBSDM buffer. Peptide samples were serially diluted in TBSDM containing 10 nM FL-E2 and incubated at room temperature for 30 min. Annealed oligonucleotides were end labeled for 30 min at room temperature in the presence of 16 nM (each) dATP, dTTP, and dGTP plus 16 nM Eu-dCTP and 0.017 U of Klenow enzyme/μl. Eu-DNA (100 pM) was then added to each FL-E2 sample and incubated at room temperature for an additional 30 min. The Eu-DNA-FL-E2 reaction mixtures were transferred into the monoclonal antibody-coated wells and incubated at room temperature for 1 h. Each well was then washed, and 100 μl of TRF enhancement solution (EG & G Wallac, Gaithersburg, Md.) was added and incubated for 10 min at room temperature. Bound Eu-DNA fluorescence was then determined on a Victor 1420 multiple label counter (EG & G Wallac). Data from three independent experiments were analyzed with the equation f = Vmax{1 − [x/(K + x)]}, where f is the signal from bound Eu-DNA, Vmax is the maximum signal, x is the peptide concentration, and K is the IC50 of synthetic peptides for inhibition of the FL-E2-Eu-DNA interaction.