Abstract
INTRODUCTION
Quadriceps weakness is one of the primary post-operative impairments that persist long term for patients after total knee arthroplasty (TKA). We hypothesized that early gait muscle recruitment patterns of the quadriceps and hamstrings with diminished knee performance at 3 months after surgery would be related to long-term quadriceps strength at one year after TKA.
METHODS
Twenty-one subjects who underwent primary unilateral TKA and 14 age-matched healthy controls were analyzed. At three months after TKA, the maximum voluntary isometric contraction of quadriceps and a comprehensive gait analysis were performed. Quadriceps strength was assessed again at one year after surgery.
RESULTS
Quadriceps muscle recruitment of the operated limb was greater than the non-operated limb during the loading response of gait (p=0.03), but there were no significant differences in hamstring recruitment or co-contraction between limbs (p>0.05). There were significant differences in quadriceps muscle recruitment during gait between the non-operated limb of TKA group and healthy control group (p<0.05). The TKA group showed a significant inverse relationship between one year quadriceps strength and co-contraction (r = −0.543) and hamstring muscle recruitment (r = −0.480) during loading response at 3 months after TKA.
CONCLUSIONS
The results revealed a reverse relationship where stronger patients tended to demonstrate lower quadriceps recruitment at 3 months post-surgery that was not observed in the healthy peer group. The altered neuromuscular patterns of quadriceps and hamstrings during gait may influence chronic quadriceps strength in individuals after TKA.
Keywords: Quadriceps strength, Muscle recruitment, Co-contraction, Gait, Total knee Arthroplasty
BACKGROUND
Total knee arthroplasty (TKA) is one of the most common knee orthopedic surgeries. In the United States, TKA was performed in more than 500,000 knees in the last year.[1] TKA has been successful in decreasing pain, increasing range of motion (ROM), correcting alignment of the lower extremity, and improving the functional status of patients.[2–5] Nevertheless, patients consistently demonstrate significantly lower functional ability in comparison to their age-matched healthy peers as measured by their time to negotiate stairs, the timed up and go, and the six-minute walk tests.[6]
One of the primary impairments that have been related to functional mobility in patients following TKA is quadriceps weakness of the operated limb.[7, 8] Quadriceps strength of the operated limb drastically decreases immediately after the surgery with an approximate 60% reduction observed from pre-operated strength.[9] Quadriceps muscle strength of the operated limb gradually increases to a level that is comparable to preoperative strength levels at 6–12 months, but is still considerably weaker than healthy age-matched peers.[8, 10] The persistent quadriceps weakness of the operated limb is related to both deficits of voluntary muscle activation and muscle atrophy.[11, 12] Quadriceps weakness of the operated limb was explained by deficits of voluntary activation (i.e. muscle inhibition) in the acute phase (e.g. 1 month) after TKA [9]. Yet, quadriceps muscle strength becomes more highly associated to muscle cross-sectional area by the one year mark after TKA as the deficits in voluntary activation are substantially reduced by this stage of recovery.[11, 12] Currently, there is limited evidence as to why patients who undergo TKA cannot resolve their chronic atrophy in the affected limb.
The characteristic movements and muscle recruitment patterns documented after surgery may be part of the reason why patients struggle to increase their quadriceps strength beyond the preoperative level. Patients with TKA assigned only 28% of the total support moment of the lower extremity during loading response in gait to their operated knee, which was lower than the non-operated limb and nearly half of the knee’s distribution observed in healthy age-matched peer groups.[8] This failed loading on the operated knee during loading response is associated with a stiff knee movement patterns with less knee flexion and prolonged co-contraction.[13] Generally, patients tend to utilize relatively limited quadriceps recruitment and prolonged and/or relatively high hamstring muscle recruitment.[13] Furthermore, asymmetry in loading between limbs during functional movements places greater dependency on the nonoperated limb to complete daily mobility tasks.[7, 8] The preferential reliance on the uninvolved limb could inhibit the development of more normal patterns of muscle recruitment of the operated knee. Walking is the most common activity of daily living [14] and it represents a substantial source of exposure to physical stress to the musculature surround the knee. The habitual limping of patients with TKA may limit the physical stress needed to maximize the recovery of quadriceps strength.
The purpose of this study was to investigate how the early performance of the musculature crossing the knee during loading response in gait relates to the persistent quadriceps weakness of the operated limb. First, we hypothesized that patients 3 months after TKA would demonstrate diminished knee performance during loading response as defined as limited knee flexion and external knee extensor moment, relatively small quadriceps recruitment, relatively large hamstring muscle recruitment, and greater quadriceps/hamstring co-contraction as compared to the data from a healthy matched peer group. Second, we expected that the aforementioned diminished knee performance of the operated limb at 3 months would be related to persistent quadriceps weakness in long-term follow-up. Lastly, we hypothesized that a similar relationship between knee performance during loading response and muscle strength would not be found in the age-matched healthy individuals.
METHODS
Subjects
Twenty-one patients who received unilateral TKA were recruited. Subjects were excluded if they had an evidence of: 1) musculoskeletal impairment other than the TKA which led to perceivable functional limitations during daily activity; 2) BMI more than 40; 3) blood pressure uncontrolled by medicine; 4) diabetes mellitus; 5) neoplasms; 6) neurological disorders; and 7) inadequate active range of motion to demonstrate normal gait patterns. The test sessions were divided into two sessions; 3 months and 1 year after TKA; 3 months after TKA is approximately the end of outpatient physical therapy [15], and 1 year after TKA is when most patients can reach the plateau of functional recovery.[16] Fourteen age-matched individuals who have asymptomatic native knees, were also recruited by the same exclusion criteria as a healthy control group. The first test session (3 months postoperative for TKA group) was divided into 2 days; using self-report questionnaires and active range of motion for the operated knee as well as quadriceps strength used the first day to assess the subjects’ functional status. The second day was to measure kinetics, kinematics, and electromyography (EMG) results from a comprehensive motion analysis system. The second session (one year after TKA), quadriceps strength was reassessed. All subjects signed an informed consent approved by the Human Subjects Review Board at the University of Delaware.
Clinical Assessments
Self-report questionnaires
The SF-36 was a questionnaire used to assess the patients’ health-related quality of life.[17–20] Physical Component Summary (PCS) has been used in patients after TKA to assess perceived physical function as an outcome of TKA.[3, 15, 21] The Knee Outcome Survey-Activities of Daily Living Scale (KOS-ADLS) was used to evaluate general subjects’ perceptions of knee impairments and the extent of disability during functional daily activity.[22]
Active Range of Motion of the Knee
The subjects had their operated knee active range of motion measured in the supine position using a plastic long arm goniometer as previously described.[5] Extension was also measured in supine with setting a block under the feet.[23]
Quadriceps Strength Measurement
Maximal voluntary isometric contraction (MVIC) of the quadriceps femoris muscle was measured as described in a previous publication.[24] Subjects were seated in a dynamometer (Kin-Com 500 H, Chattecx Corp.; Harrison, TN) with flexing the hip at 90° and the knee at 75°. The seat adjustments and the transducers settings were aligned with the subject’s knee joint to the axis of the dynamometer and were duplicated in the second test. Subjects performed practice sessions while contracting the muscle with 50%, 75% and 100% of their voluntary maximum contraction, to become familiar with the testing procedure and to warm-up the muscle. A maximum of 3 trials was recorded for each limb. In that case, the highest volitional force was used for analysis.
Gait Assessment
Motion Analysis
Gait Analysis was performed using a three dimensional, eight camera motion analysis system (VICON Peak, version 5.1, Oxford Metrics; London, England) with two force plates (Bertec Corp.; Worthington, OH) embedded flush with the floor for two consecutive steps during gait. The video data was sampled at 120 Hz while the analog signals from the force plates were sampled at 1080 Hz. Fourteen millimeter retro reflective markers were placed bilaterally on the lateral malleolus, lateral femoral condyle, greater trochanter, and iliac crest were used with joint thickness measures from calipers to identify joint centers. Rigid thermoplastic shells with four markers oriented orthogonally were secured bilaterally to the patients’ shanks and thighs using elastic wraps (SuperWrap TM, Fabrifoam, Inc.; Exton, PA) in order to minimize movement artifacts. Two markers located on the heel counter of the shoes with an additional marker on the fifth metatarsal tracked the three dimensional movement of the foot.
A standing calibration was performed prior to walking trials in order to identify the joint centers with respect to each segments coordinate system. Following the standing calibration, the subjects practiced walking along a 10m walkway until a consistent self-selected velocity was achieved, as measured by two photoelectric cells placed 286.5 cm apart. The force plates were located in the middle of the walkway. A total of 10 walking trials were collected whereby subjects contacted opposing force plates with each foot without targeting. Walking velocity was maintained to within 5% of average of practice trials as measured with the photoelectric cells. Trials outside of this range were excluded from the analysis.
Electromyography (EMG)
EMG was recorded at 1080 Hz with a 16 channel system (Motion Lab Systems; Baton Rouge, LA) interfaced with the VICON workstation. Active surface electrodes (input impedance of 100 mΩ, a common mode rejection ratio of 100 dB at 40 Hz, and a bandwith of 20–1000 Hz) made of surgical grade stainless steel, with parallel detection surfaces and a center to center distance of 2.03cm (disk diameter of 1.19cm), were used (Leukotape, Beiersdorf-Jobst Inc.; Rutherford College, NC). The tested muscles were the vastus lateralis of the quadriceps and the long head of biceps femoris to represent the hamstrings, and electrodes were placed at mid-muscle belly in parallel with the muscle fiber orientation.[25] Elastic bands (SuperWrapTM, Fabrifoam, Inc., Exton, PA) were wrapped over the electrodes to minimize movement artifacts of the electrode on the skin. The subjects were asked to complete MVIC tests for the each muscle in order to verify electrode placements and also to obtain the maximum signals to be used for the normalization.
Data Management
Marker trajectories were low pass filtered at 6 Hz and kinetic data from the force plate were low pass filtered at 40 Hz. The joint angles were calculated using rigid body analysis employing Euler angles, and the joint kinetics were calculated based on inverse dynamics, and they were expressed as net internal moments normalized to body weight × height (Nm) (Visual3D, Version 3.79, NIH Biomechanics Laboratory; Bethesda, MD). Loading response was defined as from 100ms prior to heel strike when the component of force plate data (Fz) exceeded 20N, to the first peak knee flexion during stance phase, and it was normalized to 100%.
Visual 3D software was used to further filter the signals using a bandpass butterworth filter at 20–350 Hz. Following a full wave rectification, a linear envelope of the signal was created through an 8th order, phase corrected Butterworth filter with a low pass cutoff frequency of 20 Hz. This linear envelope was normalized to the maximum signal obtained during the MVIC trials or during walking. EMG data collected during walking was analyzed with a custom-written software program (Labview 8, National Instruments; Austin, TX) to identify the average rectified value of muscle activity during loading response. The average of co-contraction during loading response was investigated, and was calculated using the following technique described by Rudolph et al.[26] The formula indicated an estimate of relative recruitment of the pair of two muscles as well as the magnitude of the co-contraction.
Paired t-tests were used to detect mean differences between limbs in the TKA group in order to determine the inter-limb asymmetrical/abnormal gait patterns. Independent t-tests were used to determine pathological gait patterns by comparing between the TKA group at 3 months after the surgery and the healthy group. Since there were no inter-limb differences in the healthy controls, the averaged of both limbs were applied to compare to the TKA group. The Pearson Product Moment Correlation Coefficient was applied to determine the strength of relationships between knee extensor moment, MVIC and the EMG results. Alpha level was set as 0.05 to determine significance in all statistical tests.
RESULTS
Table 1 states the results of the comparison of the clinical assessments between the TKA group and healthy controls. There were no differences in age and anthropometry results between groups (all: p>0.05) although there are some differences in physical measurements (e.g. knee ROM, muscle strength) (Table 1).
Table 1.
Subject Information.
| Unit | TKA (N=21) | Healthy Cohort (N=14) | |||
|---|---|---|---|---|---|
| 3 months | 1 year | ||||
| Age | years | 63.0±8.10 | 64.1±6.46 | ||
| Height | m | 1.71±0.08 | 1.75±0.10 | ||
| BMI | kg/m2 | 31.25±3.39 | 29.54±5.56 | ||
| SF-36 PCS | 43.70±7.67** | 52.23±5.71 | |||
| KOS-ADLS | 78%±12%** | 99%±2% | |||
| AROM | degree | extension | 1.7±2.2** | −0.5±2.5 | |
| NMVIC | flexion | 116.9±9.0** | 134.1±7.3 | ||
| operated | 16.63±6.07**‡‡ | 19.98±6.22‡‡ | 29.39±6.33 | ||
| nonoperated | 25.55±8.93* | 23.52±8.14 | |||
| Gait Speed | m/sec | 1.34±0.10 | 1.44±0.15* | ||
(p<0.05),
(p<0.01) Significant Differences between Group
(p<0.01) Significant Differences between Limbs
Quadriceps strength significantly increased in the operated limb (p=0.001)
AROM (active range of motion) was reported for only the operated limb in the TKA group, and the average of two limbs in the healthy control group.
BMI: Body mass index
SF-36 PCS: The short form-36 physical component score (norm population score = 50 ± 10)
KOS-ADLS: Knee outcome survey-activity of daily living scale (100% = best)
AROM: Active range of motion (knee)
NMVIC: Normalized maximum voluntary isometric contraction (newtons of force/BMI)
m: meters
Kg: kilograms
°: Degree
Gait speed of TKA group at 3 months after surgery was significantly slower than the healthy controls (p=0.012). There was significantly lower muscle recruitment in quadriceps of the operated limb during loading response compared to the nonoperated limb (p=0.030) at the 3 month test after TKA (Figure 1). There were no differences in muscle recruitment patterns in both quadriceps and hamstrings muscles, and their co-contraction when comparing the operated limbs and the healthy limbs (all: p>0.05; Figure 1). Muscle recruitment in quadriceps of the non-operated limb was significantly greater and the operated limb was significantly less than the response in healthy controls (p=0.002 and p=??). There is no significant difference in peak knee flexion angle between limbs in the TKA group or when comparing TKA limbs to the healthy comparison group (all: p>0.05, Figure 2). Knee flexion excursion in the operated limb was significantly lower than the non-operated limb (p<0.001) and the healthy controls (p=0.030), but there is no significant difference in knee flexion excursion between the non-operated limb and the healthy limbs (p=0.453; Figure 2). There were not significantly differences in knee extensor moment between limbs (p=0.353), yet the healthy limbs showed significantly greater than both the operated limb (p=0.004) and the non-operated limb (p=0.036) in TKA group.
Figure 1.
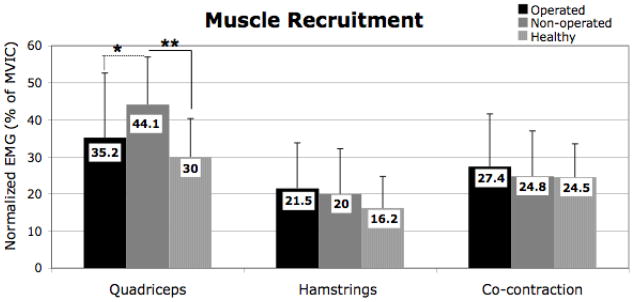
Differences in Muscle Recruitment during Loading Response (Mean ±1 Standard Deviation).
*: p<0.05, **: p<0.01M
Sold lines represent the significant differences between groups, and the dash lines represent the significant differences between limbs.
Figure 2.
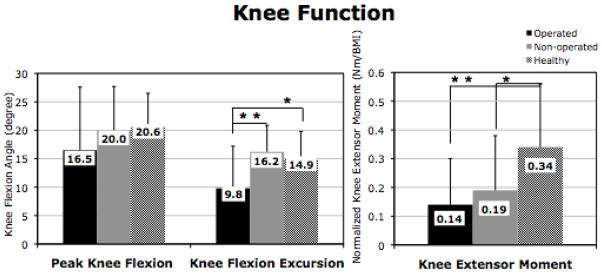
Knee Kinematics and Kinetics during Loading Response (Mean ±1 Standard Deviation).
*: p<0.05, **: p<0.01
Sold lines represent the significant differences between groups, and the dash lines represent the significant differences between limbs.
Quadriceps weakness of the operated limb 3 months after TKA was significantly related to muscle recruitment in hamstrings (r=−0.491, p=0.024) and co-contraction during loading response (r=−0.438, p=0.047: Table 2). Quadriceps weakness of the operated limb 1 year after TKA continued to be related to muscle recruitment of the hamstrings (r=−0.480, p=0.028) and co-contraction during loading response at 3 months after TKA (r=−0.543, p=0.011), yet not to muscle recruitment of the quadriceps of the operated limb (r=−0.375, p=0.094: Table 2). None of the gait characteristics of interest were related to quadriceps muscle strength in the healthy cohort. (p>0.05, Table 2).
Table 2.
Quadriceps Strength and Muscle Recruitment Patterns.
| TKA | NMIVC_op 3mos | NMVIC_op 1yr | |||
|---|---|---|---|---|---|
| Knee Function of the Operated Limb | R | p | R | p | |
| Hamstrings NEMG | % MVIC | −0.491 | 0.024* | −0.480 | 0.028* |
| Quadriceps NEMG | % MVIC | −0.071 | 0.759 | −0.375 | 0.094 |
| Co-contraction | −0.438 | 0.047* | −0.543 | 0.011* | |
| Knee Extensor Moment | Nm/BW*HT | −0.168 | 0.467 | −0.069 | 0.766 |
| Peak Knee Angle | degree | 0.312 | 0.168 | 0.154 | 0.504 |
| Knee Flexion Excrsion | degree | 0.415 | 0.061 | 0.199 | 0.386 |
| Healthy Controls | R | p | |
|---|---|---|---|
| Hamstrings Actiity | % of activity during MVIC | −0.012 | 0.967 |
| Quadriceps Activiy | % of activity during MVIC | −0.398 | 0.158 |
| Co-contraction | −0.201 | 0.490 | |
| Knee Extensor Moment | Nm/BW* HT | −0.084 | 0.775 |
| Peak Knee Angle | degree | 0.159 | 0.588 |
| Knee Flexion Excursion | degree | 0.073 | 0.804 |
Significant Relations to the Quadriceps Strength
NEMG: Normalized electromyography to the peak during strength testing
Nm: Newton * meters
BW*HT: Body weight * Height
MVIC: Maximum voluntary isometric contraction
DISCUSSION
Patients three months after TKA demonstrated an impaired loading response of the operated knee with diminished knee flexion excursion, a small knee extensor moment, and low quadriceps muscle recruitment. Such a pattern is a typical indication of failure of normal knee loading during gait in individuals with a variety of knee pathologies.[26–28] Only the muscle recruitment patterns during loading response displayed a meaningful association with long-term quadriceps strength outcomes. While co-contraction was not substantially different between limbs, or when comparing the TKA limbs to the healthy cohort, those patients with TKA who utilized more muscle co-contraction in their operated limb during loading response had less long-term quadriceps strength.
We had hypothesized that knee kinetics and kinematics would be predictive of long-term quadriceps weakness, but it was muscle recruitment patterns that were the most telling feature of future weakness in the gait pattern. Further, we had expected our finding of less quadriceps recruitment on the operated limb to relate to future muscle weakness. We posited that low muscle recruitment would limit the regional physical stress necessary to combat disuse atrophy. Our hypothesis was not supported by our data. In fact, the relationship even trended towards a reverse relationship where stronger patients tended to employ relatively smaller levels of quadriceps recruitment.
The inverse relationship between muscle recruitment and strength does have intuitive logic as a stronger muscle given a similar task demand would likely need to be recruited less to produce equivalent joint torque. In addition, the relationship is corroborated when examining the trend for an inverse relationship within the healthy cohort. The healthy cohort had much stronger quadriceps, walked faster, and had greater knee extensor moment than the TKA group, and yet their quadriceps recruitment was the lowest of the groups tested. The healthy group demonstrated no significant relationships between their muscle recruitment patterns and their quadriceps strength so this suggests that relationship between quadriceps and hamstring co-contraction is a phenomenon unique to the TKA group.
Our hypothesis regarding the negative relationship between co-contraction and quadriceps strength of the operated limb 1 year after TKA was supported. It may be difficult for patients following TKA to increase quadriceps strength by the late post-operative phase if they demonstrate greater co-contraction during loading response at 3 months after TKA. The amount of co-contraction could be from a variety of reasons; possibly quadriceps weakness, post-operated symptoms [29–31] and/or altered gait patterns that persist from the patients’ pre-operative condition.[32, 33] The unexpectedly strong inverse relationship between hamstrings muscle recruitment levels and quadriceps strength in both 3 months and 1 year after TKA may help to explain why co-contraction is correlated to weakness. The hamstring muscle recruitment should be phasing out during the start of loading response and the quadriceps recruitment should be the dominant active muscle group. Altered hamstring activity patterns during loading response have been observed among those who demonstrate abnormal functional performance after other knee injuries.[28, 34, 35] Patients with knee pathology often use the hamstrings in place of the strong quadriceps recruitment in order to both stabilizing knee with less knee flexion angle and extending the hip for forward progression.[35, 36] This strategy is beneficial as an efficient means to allow for forward progression of the body during stance.[37]
The reader is cautioned to remember that correlation does not imply causation. There may be some common underpinning factor, which underlies the relationship between persistent quadriceps weakness and quadriceps-hamstring co-contraction during gait. It should be noted that our TKA group includes people with excellent clinical outcomes as demonstrated by high self-report questionnaires, excellent operative knee range of motion, and relatively fast self-paced walking speed due to the intensive outpatient physical therapy protocols.[15] We would suggest that the relationships between gait muscle recruitment patterns and long-term strength outcomes might be stronger in more involved patients. Also, the methods used in the current study revealed a significant but moderate relationship between muscle recruitment patterns and long-term muscle strength outcomes. The addition of daily step counts, or investigating other less common tasks like stair climbing, might be another important factor to better appreciate the amount of physical exposure experienced by the quadriceps muscle with walking after knee arthroplasty.
The correlation between high co-contraction of the quadriceps and hamstring muscles during loading response of gait and quadriceps weakness could have important implications for treatment of patients with end-stage knee OA. Adapting muscle recruitment patterns after TKA with retraining to increase relative quadriceps recruitment and lessen the recruitment from the hamstring muscles during gait could assist in strength restoration efforts. In fact, a recent case report of patient who scored below norms in function with abnormal movement mechanics prior to surgery but achieved normal knee motion and moments after post-operative treatment that added movement retraining with biofeedback.[38] Avoiding excessive delay in utilizing TKA in the disease process of knee OA might also influence strength patterns via better movement patterns. Those patients who are more debilitated prior to TKA generally do not achieve as good of outcomes.[39] Some of the failure to progress may be due to long-standing and worsening patterns of co-contraction that develop prior to surgery [40, 41] and persist after knee replacement. [42–44] Continued efforts to explore the relationship between long-term muscle weakness and altered muscle recruitment patterns during daily activities are warranted in the pursuit to improve post-operative outcomes after TKA.
Footnotes
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuri Yoshida, University of Evansville, Department of Physical Therapy, 1800 Lincoln Ave, Evansville, IN, 47722.
Ryan L. Mizner, The University of Montana-Missoula, School of Physical Therapy and Rehabilitation Science, 32 Campus Drive, Missoula, MT 59812.
Lynn Snyder-Mackler, University of Delaware, Department of Physical Therapy, Graduate Program in Biomechanics and Movement Sciences and Center for Biomedical Engineering Research, 301 McKinly lab, Newark, DE 19716.
References
- 1.Losina E, Walensky RP, Kessler CL, Emrani PS, Reichmann WM, Wright EA, et al. Cost-effectiveness of total knee arthroplasty in the United States: patient risk and hospital volume. Arch Intern Med. 2009;169(12):1113–21. doi: 10.1001/archinternmed.2009.136. discussion 1121–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birdsall PD, Hayes JH, Cleary R, Pinder IM, Moran CG, Sher JL. Health outcome after total knee replacement in the very elderly. J Bone Joint Surg Br. 1999;81(4):660–2. doi: 10.1302/0301-620x.81b4.9380. [DOI] [PubMed] [Google Scholar]
- 3.Heck DA, Robinson RL, Partridge CM, Lubitz RM, Freund DA. Patient outcomes after knee replacement. Clin Orthop Relat Res. 1998;356:93–110. doi: 10.1097/00003086-199811000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Ranawat CS, Ranawat AS, Mehta A. Total knee arthroplasty rehabilitation protocol: what makes the difference? J Arthroplasty. 2003;18(3 Suppl 1):27–30. doi: 10.1054/arth.2003.50080. [DOI] [PubMed] [Google Scholar]
- 5.Mizner RL, Petterson SC, Snyder-Mackler L. Quadriceps strength and the time course of functional recovery after total knee arthroplasty. J Orthop Sports Phys Ther. 2005;35(7):424–36. doi: 10.2519/jospt.2005.35.7.424. [DOI] [PubMed] [Google Scholar]
- 6.Farquhar S, Snyder-Mackler L. The Chitranjan Ranawat Award: The Nonoperated Knee Predicts Function 3 Years after Unilateral Total Knee Arthroplasty. Clin Orthop Relat Res. 2009 doi: 10.1007/s11999-009-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizner RL, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res. 2005;23(5):1083–90. doi: 10.1016/j.orthres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin Biomech (Bristol, Avon) 2008;23(3):320–8. doi: 10.1016/j.clinbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty. The contributions of muscle atrophy and failure of voluntary muscle activation. J Bone Joint Surg Am. 2005;87(5):1047–53. doi: 10.2106/JBJS.D.01992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meier W, Mizner RL, Marcus RL, Dibble LE, Peters C, Lastayo PC. Total knee arthroplasty: muscle impairments, functional limitations, and recommended rehabilitation approaches. J Orthop Sports Phys Ther. 2008;38(5):246–56. doi: 10.2519/jospt.2008.2715. [DOI] [PubMed] [Google Scholar]
- 11.Petterson S, Barrance P, Marmon A, Handling T, Buchanan T, Snyder-Mackler L. Time Course of Quad Strength, Area and Activation After Knee Arthroplasty and Strength Training. Med Sci Sports Exerc. 2010 doi: 10.1249/MSS.0b013e3181eb639a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier WA, Marcus RL, Dibble LE, Foreman KB, Peters CL, Mizner RL, et al. The long-term contribution of muscle activation and muscle size to quadriceps weakness following total knee arthroplasty. J Geriatr Phys Ther. 2009;32(2):35–8. [PubMed] [Google Scholar]
- 13.Benedetti MG, Catani F, Bilotta TW, Marcacci M, Mariani E, Giannini S. Muscle activation pattern and gait biomechanics after total knee replacement. Clin Biomech (Bristol, Avon) 2003;18(9):871–6. doi: 10.1016/s0268-0033(03)00146-3. [DOI] [PubMed] [Google Scholar]
- 14.Tudor-Locke CE, Myers AM. Challenges and opportunities for measuring physical activity in sedentary adults. Sports Med. 2001;31(2):91–100. doi: 10.2165/00007256-200131020-00002. [DOI] [PubMed] [Google Scholar]
- 15.Petterson SC, Mizner RL, Stevens JE, Raisis L, Bodenstab A, Newcomb W, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61(2):174–83. doi: 10.1002/art.24167. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy DM, Stratford PW, Riddle DL, Hanna SE, Gollish JD. Assessing recovery and establishing prognosis following total knee arthroplasty. Phys Ther. 2008;88(1):22–32. doi: 10.2522/ptj.20070051. [DOI] [PubMed] [Google Scholar]
- 17.Keller SD, Ware JE, Jr, Gandek B, Aaronson NK, Alonso J, Apolone G, et al. Testing the equivalence of translations of widely used response choice labels: results from the IQOLA Project. International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):933–44. doi: 10.1016/s0895-4356(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 18.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 19.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 21.Moffet H, Collet JP, Shapiro SH, Paradis G, Marquis F, Roy L. Effectiveness of intensive rehabilitation on functional ability and quality of life after first total knee arthroplasty: A single-blind randomized controlled trial. Arch Phys Med Rehabil. 2004;85(4):546–56. doi: 10.1016/j.apmr.2003.08.080. [DOI] [PubMed] [Google Scholar]
- 22.Irrgang JJ, Snyder-Mackler L, Wainner RS, Fu FH, Harner CD. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80(8):1132–45. doi: 10.2106/00004623-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Gogia PP, Braatz JH, Rose SJ, Norton BJ. Reliability and validity of goniometric measurements at the knee. Phys Ther. 1987;67(2):192–5. doi: 10.1093/ptj/67.2.192. [DOI] [PubMed] [Google Scholar]
- 24.Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21(5):775–9. doi: 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 25.Kendall FP, McCreary EK, Provance PG. Muscle testing and function. Philadelphia, PA: Williams and Wilkins; 1993. [Google Scholar]
- 26.Rudolph KS, Snyder-Mackler L. Effect of dynamic stability on a step task in ACL deficient individuals. J Electromyogr Kinesiol. 2004;14(5):565–75. doi: 10.1016/j.jelekin.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Schmitt LC, Rudolph KS. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arthritis Rheum. 2007;57(6):1018–26. doi: 10.1002/art.22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chmielewski TL, Rudolph KS, Snyder-Mackler L. Development of dynamic knee stability after acute ACL injury. J Electromyogr Kinesiol. 2002;12(4):267–74. doi: 10.1016/s1050-6411(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 29.Brander VA, Stulberg SD, Adams AD, Harden RN, Bruehl S, Stanos SP, et al. Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res. 2003;416:27–36. doi: 10.1097/01.blo.0000092983.12414.e9. [DOI] [PubMed] [Google Scholar]
- 30.Dahlen L, Zimmerman L, Barron C. Pain perception and its relation to functional status post total knee arthroplasty: a pilot study. Orthop Nurs. 2006;25(4):264–70. doi: 10.1097/00006416-200607000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Dennis DA. Evaluation of painful total knee arthroplasty. J Arthroplasty. 2004;19(4 Suppl 1):35–40. doi: 10.1016/j.arth.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 32.Astephen JL, Deluzio KJ, Caldwell GE, Dunbar MJ. Biomechanical changes at the hip, knee, and ankle joints during gait are associated with knee osteoarthritis severity. J Orthop Res. 2008;26(3):332–41. doi: 10.1002/jor.20496. [DOI] [PubMed] [Google Scholar]
- 33.Lewek MD, Rudolph KS, Snyder-Mackler L. Control of frontal plane knee laxity during gait in patients with medial compartment knee osteoarthritis. Osteoarthritis Cartilage. 2004;12(9):745–51. doi: 10.1016/j.joca.2004.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hurd WJ, Snyder-Mackler L. Knee instability after acute ACL rupture affects movement patterns during the mid-stance phase of gait. J Orthop Res. 2007;25(10):1369–77. doi: 10.1002/jor.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacWilliams BA, Wilson DR, DesJardins JD, Romero J, Chao EY. Hamstrings cocontraction reduces internal rotation, anterior translation, and anterior cruciate ligament load in weight-bearing flexion. J Orthop Res. 1999;17(6):817–22. doi: 10.1002/jor.1100170605. [DOI] [PubMed] [Google Scholar]
- 36.Neptune RR, Zajac FE, Kautz SA. Muscle force redistributes segmental power for body progression during walking. Gait Posture. 2004;19(2):194–205. doi: 10.1016/S0966-6362(03)00062-6. [DOI] [PubMed] [Google Scholar]
- 37.Winter DA. Knee flexion during stance as a determinant of inefficient walking. Phys Ther. 1983;63(3):331–3. doi: 10.1093/ptj/63.3.331. [DOI] [PubMed] [Google Scholar]
- 38.McClelland J, Zeni J, Haley RM, Snyder-Mackler L. Functional and biomechanical outcomes after using biofeedback for retraining symmetrical movement patterns after total knee arthroplasty: a case report. J Orthop Sports Phys Ther. 2012;42(2):135–44. doi: 10.2519/jospt.2012.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fortin PR, Penrod JR, Clarke AE, St-Pierre Y, Joseph L, Belisle P, et al. Timing of total joint replacement affects clinical outcomes among patients with osteoarthritis of the hip or knee. Arthritis Rheum. 2002;46(12):3327–30. doi: 10.1002/art.10631. [DOI] [PubMed] [Google Scholar]
- 40.Hubley-Kozey CL, Hill NA, Rutherford DJ, Dunbar MJ, Stanish WD. Co-activation differences in lower limb muscles between asymptomatic controls and those with varying degrees of knee osteoarthritis during walking. Clin Biomech (Bristol, Avon) 2009;24(5):407–14. doi: 10.1016/j.clinbiomech.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Rutherford DJ, Hubley-Kozey CL, Stanish WD, Dunbar MJ. Neuromuscular alterations exist with knee osteoarthritis presence and severity despite walking velocity similarities. Clin Biomech (Bristol, Avon) 2011;26(4):377–83. doi: 10.1016/j.clinbiomech.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Mandeville D, Osternig LR, Chou LS. The effect of total knee replacement surgery on gait stability. Gait Posture. 2008;27(1):103–9. doi: 10.1016/j.gaitpost.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Myles CM, Rowe PJ, Walker CR, Nutton RW. Knee joint functional range of movement prior to and following total knee arthroplasty measured using flexible electrogoniometry. Gait Posture. 2002;16(1):46–54. doi: 10.1016/s0966-6362(01)00198-9. [DOI] [PubMed] [Google Scholar]
- 44.Smith AJ, Lloyd DG, Wood DJ. A kinematic and kinetic analysis of walking after total knee arthroplasty with and without patellar resurfacing. Clin Biomech (Bristol, Avon) 2006;21(4):379–86. doi: 10.1016/j.clinbiomech.2005.11.007. [DOI] [PubMed] [Google Scholar]


