Table 1.
Structure activity of phenylheterocyclic N-terminal Partial Ligand Alternatives
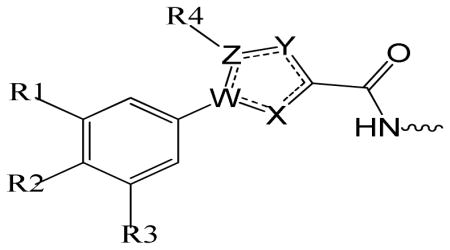
| |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCCP ID | R1 | R2 | R3 | R4 | W | X | Y | Z | CDK2/cyclin A IC50 (μM) |
CDK4/cyclin D1 IC50 (μM) |
|
| Triazole | 5843 | H | H | H | H | N | N | N | C | 16.2±3 | 49.2 ±7.60 |
| 5773 | Cl | H | Cl | CH3 | N | N | N | C | 4.0±0.6 | 27.3 ± 3.40 | |
| 5774 | H | Cl | H | CH3 | N | N | N | C | 11.5±3.3 | 12.0 ± 2.06 | |
| 5906 | Cl | H | H | CH3 | N | N | N | C | 5.7±0.99 | 26.5±7.49 | |
| 5907 | CH3 | H | H | CH3 | N | N | N | C | 13.8±1.13 | 51.05±16.05 | |
| Pyrazole | 5762 | H | H | H | CH3 | N | N | C | C | 40.3±6.5 | 54.2 ± 3.05 |
| 5763 | Cl | H | Cl | CH3 | N | N | C | C | 21.8±13.7 | >100 | |
| 5764 | Cl | H | H | CH3 | N | N | C | C | 11.9±2.0 | 70.3 ±12.79 | |
| 5771 | F | H | H | CH3 | N | N | C | C | 29.6±12.2 | 65.9±6.60 | |
| 5765 | H | Cl | H | CH3 | N | N | C | C | 33.7±8.1 | 54.7 ±20.43 | |
| 5766 | OCH3 | H | H | CH3 | N | N | C | C | 64.1±4.2 | >100 | |
| 5767 | H | OCH3 | H | CH3 | N | N | C | C | >180 | >180 | |
| Pyrrole | 5776 | H | Cl | H | H | N | C | C | C | >180 | >180 |
| 5775 | Cl | H | Cl | H | N | C | C | C | >180 | >180 | |
| Furan | 5768 | Cl | H | Cl | H | C | O | C | C | >180 | >180 |
| 5769 | Cl | H | H | H | C | O | C | C | >180 | >180 | |
| Imidazole | 5760 | H | H | H | CH3 | C | N | C | N | >180 | >180 |
| 5852 | F | H | H | H | C | N | C | N | 34.3±0.6 | 68.5 ±14.84 | |
| Thiazole | 5583 | H | Cl | H | H | C | N | C | S | >180 | >180 |
