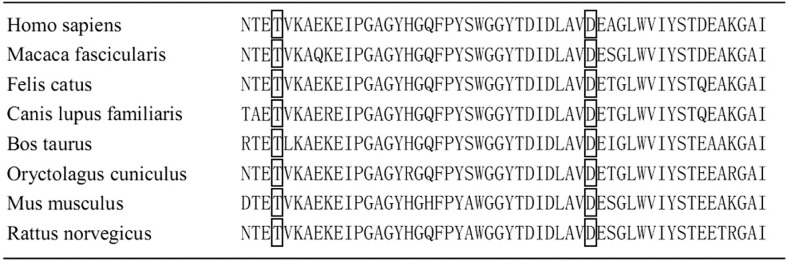Abstract
AIM
To detect the mutations in two candidate genes, myocilin (MYOC) and cytochrome P450 1B1 (CYP1B1), in a Chinese family with primary open angle glaucoma (POAG).
METHODS
The family was composed of three members, the parents and a daughter. All members of the family underwent complete ophthalmologic examinations. Exons of MYOC and CYP1B1 genes were screened for sequence alterations by polymerase chain reaction (PCR) and direct DNA sequencing.
RESULTS
The mother was the proband, she was diagnosed as POAG in both eyes. Her daughter was diagnosed as juvenile-onset POAG. The father was asymptomatic. One MYOC heterozygous mutation c.1150 G>A (D384N) in exon 3 was identified in the mother, another MYOC heterozygous variation c.1058 C>T (T353I) in exon 3 was identified in the father, and the daughter inherited both of the variations. Meanwhile, three single nucleotide polymorphisms (SNPs) in CYP1B1 gene were found in the family.
CONCLUSION
The D384N mutation of MYOC has been reported as one of disease-causing mutations in POAG, whereas T353I variation of MYOC was thought as a high risk factor for POAG. The two variations of MYOC were first reported in one juvenile-onset POAG patient who presented with more severe clinical manifestations, suggesting that T353I polymorphism of MYOC may be associated with the severity of POAG.
Keywords: primary open angle glaucoma, myocilin, mutation, D384N, T353I
INTRODUCTION
Glaucoma is one of the leading causes of blindness in the world, and is typically characterized by high intraocular pressure (IOP), optic disc cupping and visual field defects. Increased IOP, the major risk factor of this disease, causes irreversible damage to the optic nerve and leads to blindness if untreated. There are several types of primary glaucoma, with primary open angle glaucoma (POAG) being the most common form of this ocular disease [1]. To date, six genes, myocilin (MYOC), cytochrome P450 1B1 (CYP1B1), optineurin (OPTN), WD repeat domain 36 (WDR36), neurotrophin 4 (NTF4), and latent transforming growth factor beta binding protein 2 (LTBP2) have been identified as primary glaucoma-causing genes[2]. MYOC is the first gene identified to be responsible for POAG [3], [4]. The MYOC gene has three exons and most mutations were found in the third exon which encodes the olfactomedin-like domain [5]. CYP1B1 is associated with primary congenital glaucoma (PCG)[6], POAG and other forms of glaucoma including Peter's anomaly and Axenfeld-Rieger syndrome[2]. Over 80 glaucoma-associated mutations in CYP1B1 have been described [7].
In this study, we detected MYOC and CYP1B1 genes for a POAG family, and identified a known disease-causing mutation (c.1150 G>A, D384N) and a high-risk variation (c.1058C>T, T353I) in exon 3 of MYOC in one patient who presented more severe phenotype than her mother carrying only D384N mutation. It is the first time, to the best of our knowledge, these two heterozygous sequence variations in the same exon of MYOC were found in one patient with juvenile-onset POAG.
SUBJECTS AND METHODS
Subjects
This family with POAG (Figure 1) was recruited from the out-patient department of Ophthalmology at West China Hospital (Sichuan University, Chengdu, China). No consanguineous marriage was noticed in the family. The study was approved by the medical ethics committee of the West China Hospital of Sichuan University. Informed consent was obtained from all participants according to the principles of Declaration of Helsinki.
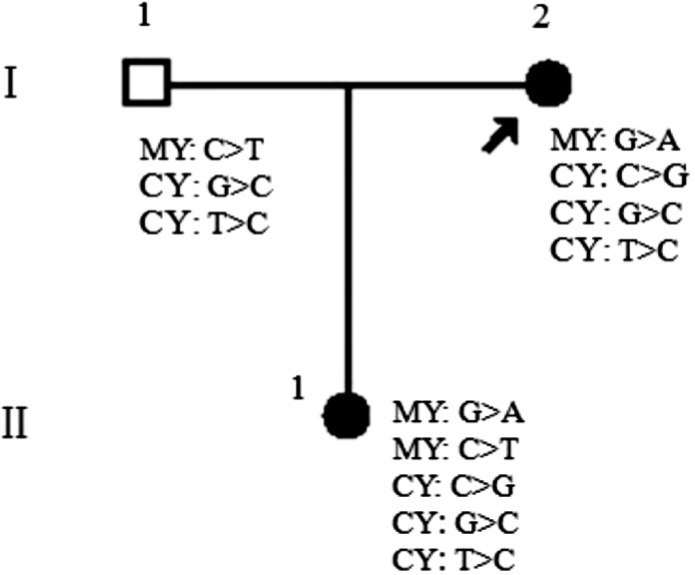
Figure 1. Pedigree of the Chinese glaucoma family.
The variations of MYOC and CYP1B1 genes were summarized in the figure of the family. The MYOC heterozygous pathogenic mutation, G>A (D384N) was identified in two patients but not in the asymptomatic member. Another MYOC heterozygous variation, C>T (T353I) was observed in the unaffected father and patient (II:1). Besides, one C>G (R48G) variation in exon 2 of CYP1B1 was found only in two patients. G>C (V432L) variation and T>C (D449D) variation in exon 3 of CYP1B1, were identified in all patients and asymptomatic member. All these indicated that T353I variation appeared to be involved in the difference of disease phenotype between the patients. Abbreviations: MY, MYOC; CY, CYP1B1. Arrow indicates the proband.
Methods
Clinical examination
All members of the family underwent complete ophthalmologic examinations including visual acuity, slit-lamp biomicroscopy, applanation tonometry, gonioscopy, funduscopy and perimetry. POAG was defined by a normal appearing anterior chamber angle along with three of the following symptoms: elevation of intraocular pressure (IOP>21mmHg), characteristic glaucomatous visual field defects and optic disc damage. Individuals without any of the manifestations were defined as asymptomatic ones. All members were clinically evaluated by glaucoma specialists.
Molecular genetic analysis
Peripheral blood was collected for DNA analysis from each individual involved in this study. Genomic DNA was extracted from peripheral blood leukocytes using QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany) by standard protocols. The coding sequences of MYOC (GenBank AB006688) and CYP1B1 (GenBank U56438) were amplified by polymerase chain reaction (PCR) using a MyCycler thermocycler (Bio-Rad, Hercules, CA). Each 30µL PCR amplification reaction mixture contained 30ng genomic DNA, 1.0µmol/L of each of the forward and reverse primers (Table 1) and 15µL of 2×Taq Master Mix (SinoBio Biltech Co., Ltd, Shanghai, China). PCR conditions were as follows: initial step of denaturation at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at a temperature specific for 30 seconds, and extension at 72°C for 1-3 minutes, and then a final extension at 72°C for 5 minutes. The resulting PCR products were purified with a cycle-pure kit (OMEGA; Bio-Tek, Doraville, GA) and sequenced on the ABI 3730XL automated DNA sequencer (Applied Biosystems, Foster City, CA). The DNA sequences obtained from the sequencing were compared with the published MYOC and CYP1B1 sequences.
Table 1. Primers used in PCR for amplification of MYOC and CYP1B1.
| Exons | Primer sequence (forward/reverse) | Product size (bp) |
| MYOC 1 | PF5′-CCAAACAGACTTCTGGAAGG-3′ | 904 |
| MYOC 1 | PR 5′-TAGCAGGTCACTACGAGCC-3′ | |
| MYOC 2 | PF 5′-TGTCATCCTCAACATAGTCA-3′ | 351 |
| MYOC 2 | PR 5′-TTCTGTTCCTCTTCTCCTC-3′ | |
| MYOC 3 | PF 5′-CCAGGGCTGTCACATCTACT-3′ | 933 |
| MYOC 3 | PR 5′-CATCTCCTTCTGCCATTGC-3′ | |
| CYP1B1 2 | PF 5′-CATTTCTCCAGAGAGTCAGC-3′ | 1260 |
| CYP1B1 2 | PR5′-GCTTGCAAACTCAGCATATTC-3′ | |
| CYP1B1 3 | PF5′-ACCCAATGGAAAAGTCAGCC-3′ | 927 |
| CYP1B1 3 | PR 5′-GCTTGCCTCTTGCTTCTTATT-3′ |
RESULTS
Clinical findings
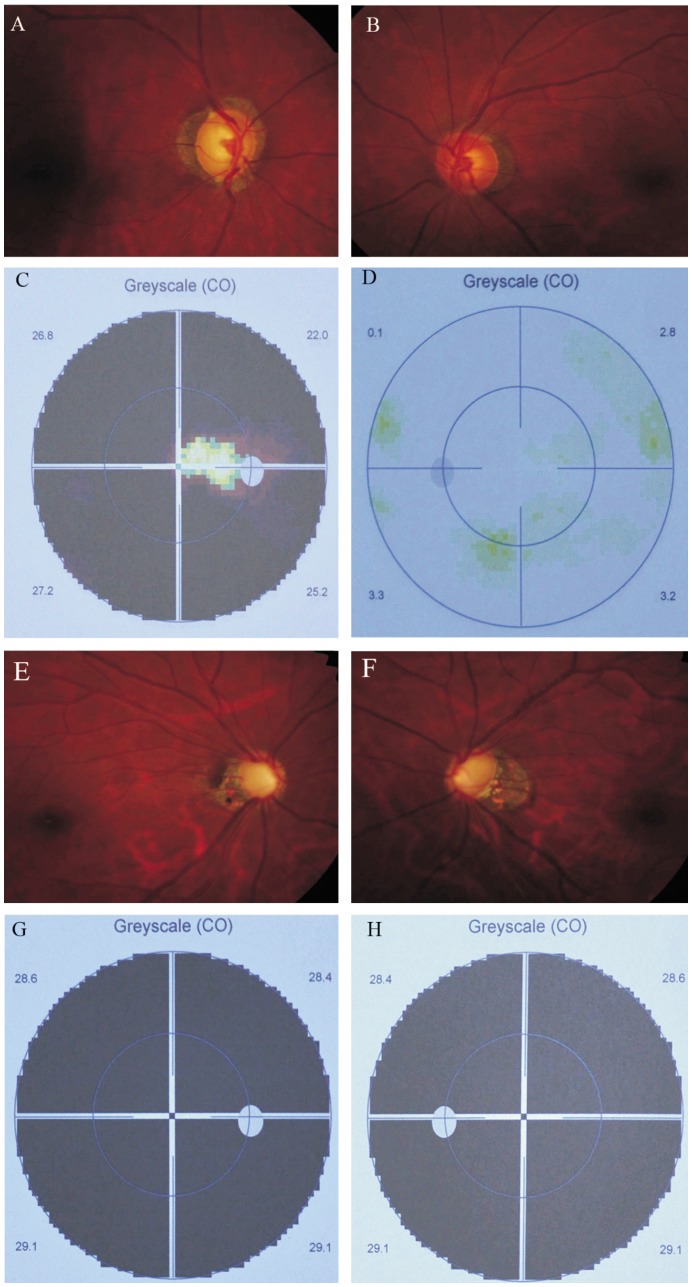
This family was composed of the parents and a daughter (Table 2). The mother (I:2) who was firstly diagnosed as POAG in both eyes at the age of 54, presented with elevated IOP (28mmHg in the both eyes), open anterior chamber angle, enlarged cup-disc ratio of 0.9/0.5(OD/OS) and characteristic glaucomatous visual field defects (Figure 2). Other ocular abnormalities or systemic disorders were not found. The IOP of the proband was well controlled with anti-glaucoma eye drops such as alphagan, latanoprost and brinzolamide. The daughter (II:1) was diagnosed as juvenile-onset POAG in both eyes at the age of 29. A cup-disc ratio of 0.95/0.95 (OD/OS), IOP at 32/31mmHg (OD/OS) and late-stage glaucomatous visual field loss were observed (Figure 2). She failed to response well to anti-glaucoma medication as mentioned and selective laser trabeculoplasty (SLT).
Table 2. Clinical features of the POAG patients.
| Patients | Gender | Diagnosis age (a) | Treatment | Maximal IOP (mmHg) | Cup-disc ratio | Central corneal thickness (µm) | Visual field damage |
| I:2 | Female | 54 | Medication | 28/28 (OD/OS) | 0.9/0.5 (OD/OS) | 489/496 (OD/OS) | Moderate |
| II:1 | Female | 29 | Medication and SLT | 32/31 (OD/OS) | 0.95/0.95 (OD/OS) | 530/528 (OD/OS) | Severe |
Figure 2. Clinical manifestations of the proband (I:2) and her daughter (II:1).
Optic disc (A, B) and visual field (C, D) of the proband. Glaucomatous optic disc atrophy was observed with visual field defects in the right eye; Optic disc (E, F) and visual field (G, H) of the proband' daughter. Glaucomatous optic disc atrophy was found with visual field defects in both eyes.
Sequencing results of MYOC and CYP1B1
In order to detect the genetic defects of the family, we screened three exons of MYOC (exon 1, 2 and 3). The asymptomatic father was also analyzed as a control. With the sequence analysis results, we identified one novel mutation D384N (c.1150 G>A) in exon 3 of MYOC in both patients (I:2, II:1) (Figure1, 3). This mutation was previously reported to be responsible for the pathogenesis of POAG [8]. Meantime, another variation T353I (c.1058 C>T) in exon 3 of MYOC, which was considered as a high risk factor for POAG [9], was identified in the asymptomatic father (I:1) and his daughter (II:1) (Figure1, 4). Through bioinformatics analysis, both of the two mutational sites were found completely conserved in 8 different species by using the online Clustalw tool (Figure 5). The variations of these sites may affect the function of the protein. Besides, three SNPs (R48G, V432L and D449D) were identified respectively in exon 2 and exon 3 of CYP1B1 (Figure 1). R48G was found only in the patients with POAG, V432L and D449D were found in both of the patients and asymptomatic father.
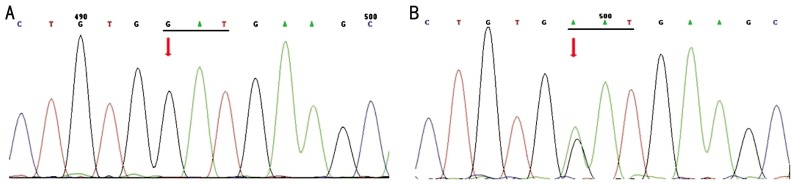
Figure 3. D384N mutation of MYOC in the POAG family.
A: Wild type sequence from the normal control (I:1); B: Arrow indicates a heterozygous mutation consisting of a G>A transversion at codon 384. This mutation was observed in both patients (I:2, II:1).
Figure 4. T353I variation of MYOC in the POAG family.
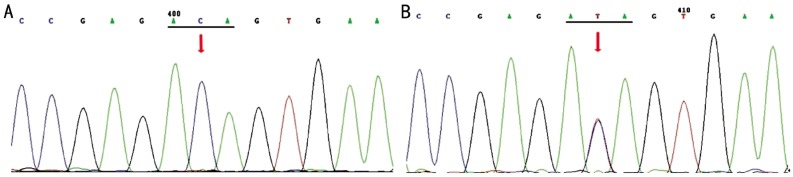
A: No T353I variation was identified in the proband (I:2); B: Arrow indicates a heterozygous variation consisting of a C>T transversion at codon 353. This variation was observed in the unaffected father (I:1) and patient (II:1).
Figure 5. The variations involved a highly conserved residue.
DISCUSSION
MYOC is the first gene identified for POAG [3], [4]. Previous studies have shown that MYOC mutations account for 3%-5% of POAG patients worldwide [2],[10]. More than 80 mutations in MYOC have been reported (http://www.myocilin.com/variants.php), 90% of which occur within exon 3. The MYOC mutations in POAG were found in different ethnicity such as Chinese, French, Spanish, American, Australian, Canadian, Indian, Swiss and Japanese [8],[11]-[18].
In this study, a heterozygous mutation consisting of a G>A transversion in exon 3 of MYOC was identified in both patients, resulting in an amino acid substitution from aspartic acid (ASP) to asparagine (ASN) at codon 384 (D384N). Jia et al [8] firstly identified this novel D384N mutant in a Chinese juvenile-onset open angle glaucoma (JOAG) family. D384N mutation hasn't been found in patients with glaucoma in any other ethnicity until now.
Another variation, T353I in exon 3 of MYOC, was identified in the daughter and her asymptomatic father. Previous studies showed that T353I variation was also found in POAG patients in different populations, such as Japanese, Korean and Indian [14], [19], [20]. Although T353I variation was previously reported as a possible POAG causing mutation, it was identified in the unaffected control and normal individuals [19], [21]. Fan et al [22] screened the SNPs of MYOC in Chinese Han population and found significant differences in frequencies of the genotype TC and the allele T of T353I between unrelated POAG patients and control individuals. T353I variation of MYOC was found more in POAG patients than in the controls [9]. Therefore, this variation may be not a disease-causing mutation, instead, it may be a risk factor for POAG. T353I variation of MYOC might increase the risk of POAG. Fan et al [9] and Wang et al [23] showed that risk factors for POAG included family history, hypertension, cigarette smoking and T353I mutation of MYOC gene.
It should be noted that the mother (patient I:2) who carried only the D384N mutation of MYOC was found to suffer from glaucoma at age of 54. She presented with elevated IOP at 28 mmHg in both eyes, enlarged cup-disc ratio of 0.9/0.5 (OD/OS) and characteristic glaucomatous visual field loss in her right eye. Her intraocular pressure can be controlled through anti-glaucoma medications. However, her daughter (patient II:1) who had both of the D384N and T353I variations of MYOC was found to suffer from glaucoma at age of 29. She presented with elevated IOP at 32/31 mmHg (OD/OS), a cup-disc ratio of 0.95/0.95 (OD/OS), and late-stage glaucomatous visual field loss in both eyes. Compared to her mother, she failed to response well to IOP lowering drops and SLT. The father who carried only T353I variation of MYOC was not clinically affected. Obviously, the compound heterozygote patient in this family who had both T353I and D384N variations in exon 3 of MYOC presented with more severe phenotype, and most likely, earlier onset age. A similar case was reported in an India POAG patient [24]. Two heterozygous sequence variants, T353I and N480K, in the same exon (exon 3) of MYOC were observed in this India patient who also had a severe disease phenotype. The presence of two variants in different exons of MYOC gene in one patient have been reported before, and the compound R126W/K423E carrier with POAG also displayed severely affected visual field and an early onset age[25]. Similar to T353I, the R126W of MYOC has been observed in controls as well. D384N, N480K and K423E are glaucoma-causing mutations. These three cases were about the compound heterozygote individual with a polymorphism and disease-causing mutation, suggesting that certain single nucleotide polymorphism can worsen the disease when combine with disease-causing mutation of the same gene. Presence of compound heterozygous variants in the same gene and the presence of a severe ocular abnormality imply a combinatorial role of the two variants of one gene in eye disease causation [24]-[26]. In this family, the D384N mutation of MYOC was deemed to largely contribute to pathogenesis of POAG, and T353I polymorphism appeared to be involved in the severity of disease phenotype and probably, the disease onset age, even though it alone may not cause POAG.
In conclusion, the D384N mutation of MYOC appears to be the cause of the disease in this family, and T353I polymorphism of MYOC increases the risk of POAG and aggravates the disorder. This is the first time that these two variations in the same exon of MYOC were found in one patient with juvenile-onset POAG. This study enhanced our understanding of the role of MYOC in pathogenesis of POAG. Obviously, further studies are needed to elucidate the role of SNP T353I, and how these two variations act together, in the pathogenesis of POAG.
Acknowledgments
Authors would like to thank all the family members for their participation and cooperation in this study.
Footnotes
Foundation item: National Nature Science Foundation of China (No.81170851)
REFERENCES
- 1.McDonald KK, Abramson K, Beltran MA, Ramirez MG, Alvarez M, Ventura A, Santiago-Turla C, Schmidt S, Hauser MA, Allingham RR. Myocilin and optineurin coding variants in Hispanics of Mexican descent with POAG. J Hum Genet. 2010;55(10):697–700. doi: 10.1038/jhg.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Allingham RR. Molecular genetics in glaucoma. Exp Eye Res. 2011;93(4):331–339. doi: 10.1016/j.exer.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat Genet. 1993;4(1):47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- 4.Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275(5300):668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 5.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360(11):1113–1124. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakrabarti S, Ghanekar Y, Kaur K, Kaur I, Mandal AK, Rao KN, Parikh RS, Thomas R, Majumder PP. A polymorphism in the CYP1B1 promoter is functionally associated with primary congenital glaucoma. Hum Mol Genet. 2010;19(20):4083–4090. doi: 10.1093/hmg/ddq309. [DOI] [PubMed] [Google Scholar]
- 7.Vasiliou V, Gonzalez FJ. Role of CYP1B1 in glaucoma. Annu Rev Pharmacol Toxicol. 2008;48:333–358. doi: 10.1146/annurev.pharmtox.48.061807.154729. [DOI] [PubMed] [Google Scholar]
- 8.Jia LY, Gong B, Pang CP, Huang Y, Lam DS, Wang N, Yam GH. Correction of the disease phenotype of myocilin-causing glaucoma by a natural osmolyte. Invest Ophthalmol Vis Sci. 2009;50(8):3743–3749. doi: 10.1167/iovs.08-3151. [DOI] [PubMed] [Google Scholar]
- 9.Fan BJ, Leung YF, Wang N, Lam SC, Liu Y, Tam OS, Pang CP. Genetic and environmental risk factors for primary open-angle glaucoma. Chin Med J (Engl) 2004;117(5):706–710. [PubMed] [Google Scholar]
- 10.Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res. 2009;88(4):837–844. doi: 10.1016/j.exer.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adam MF, Belmouden A, Binisti P, Brezin AP, Valtot F, Bechetoille A, Dascotte JC, Copin B, Gomez L, Chaventre A, Bach JF, Garchon HJ. Recurrent mutations in a single exon encoding the evolutionarily conserved olfactomedin-homology domain of TIGR in familial open-angle glaucoma. Hum Mol Genet. 1997;6(12):2091–2097. doi: 10.1093/hmg/6.12.2091. [DOI] [PubMed] [Google Scholar]
- 12.Mansergh FC, Kenna PF, Ayuso C, Kiang AS, Humphries P, Farrar GJ. Novel mutations in the TIGR gene in early and late onset open angle glaucoma. Hum Mutat. 1998;11(3):244–251. doi: 10.1002/(SICI)1098-1004(1998)11:3<244::AID-HUMU10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Morissette J, Clepet C, Moisan S, Dubois S, Winstall E, Vermeeren D, Nguyen TD, Polansky JR, Cote G, Anctil JL, Amyot M, Plante M, Falardeau P, Raymond V. Homozygotes carrying an autosomal dominant TIGR mutation do not manifest glaucoma. Nat Genet. 1998;19(4):319–321. doi: 10.1038/1203. [DOI] [PubMed] [Google Scholar]
- 14.Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K, Hoh ST, Buys YM, Dickinson J, Hockey RR, Williams-Lyn D, Trope G, Kitazawa Y, Ritch R, Mackey DA, Alward WL, Sheffield VC, Stone EM. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8(5):899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 15.Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, Williams-Lyn D, Heon E. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70(2):448–460. doi: 10.1086/338709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanagavalli J, Krishnadas SR, Pandaranayaka E, Krishnaswamy S, Sundaresan P. Evaluation and understanding of myocilin mutations in Indian primary open angle glaucoma patients. Mol Vis. 2003;9:606–614. [PubMed] [Google Scholar]
- 17.Iliev ME, Bodmer S, Gallati S, Lanz R, Sturmer J, Katsoulis K, Wolf S, Trittibach P, Sarra GM. Glaucoma phenotype in a large Swiss pedigree with the myocilin Gly367Arg mutation. Eye (Lond) 2008;22(7):880–888. doi: 10.1038/sj.eye.6702745. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi F, Suzuki Y, Shirato S, Araie M. The Gly367Arg mutation in the myocilin gene causes adult-onset primary open-angle glaucoma. Jpn J Ophthalmol. 2000;44(4):445–448. doi: 10.1016/s0021-5155(00)00201-x. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Suh W, Park SC, Kim CY, Park KH, Kook MS, Kim YY, Kim CS, Park CK, Ki CS, Kee C. Mutation spectrum of CYP1B1 and MYOC genes in Korean patients with primary congenital glaucoma. Mol Vis. 2011;17:2093–2101. [PMC free article] [PubMed] [Google Scholar]
- 20.Bhattacharjee A, Acharya M, Mukhopadhyay A, Mookherjee S, Banerjee D, Bandopadhyay AK, Thakur SK, Sen A, Ray K. Myocilin variants in Indian patients with open-angle glaucoma. Arch Ophthalmol. 2007;125(6):823–829. doi: 10.1001/archopht.125.6.823. [DOI] [PubMed] [Google Scholar]
- 21.Fan BJ, Wang DY, Fan DS, Tam PO, Lam DS, Tham CC, Lam CY, Lau TC, Pang CP. SNPs and interaction analyses of myocilin, optineurin, and apolipoprotein E in primary open angle glaucoma patients. Mol Vis. 2005;11:625–631. [PubMed] [Google Scholar]
- 22.Fan B, Liang X, Peng Z, Dong X, Liu Y, Luan J, Wang N. Study on single nucleotide polymorphism of TIGR gene in primary open-angle glaucoma patients. Zhonghua Yi xue Zazhi. 2002;82(11):743–747. [PubMed] [Google Scholar]
- 23.Wang N, Peng Z, Fan B, Liu Y, Dong X, Liang X, Luan J. Case control study on the risk factors of primary open angle glaucoma in China. Zhonghua Liuxingbingxue Zazhi. 2002;23(4):293–296. [PubMed] [Google Scholar]
- 24.Rose R, Balakrishnan A, Muthusamy K, Arumugam P, Shanmugam S, Gopalswamy J. Myocilin mutations among POAG patients from two populations of Tamil Nadu, South India, a comparative analysis. Mol Vis. 2011;17:3243–3253. [PMC free article] [PubMed] [Google Scholar]
- 25.Faucher M, Anctil JL, Rodrigue MA, Duchesne A, Bergeron D, Blondeau P, Cote G, Dubois S, Bergeron J, Arseneault R, Morissette J, Raymond V. Founder TIGR/myocilin mutations for glaucoma in the Quebec population. Hum Mol Genet. 2002;11(18):2077–2090. doi: 10.1093/hmg/11.18.2077. [DOI] [PubMed] [Google Scholar]
- 26.Chiang PW, Wang J, Chen Y, Fu Q, Zhong J, Yi X, Wu R, Gan H, Shi Y, Barnett C, Wheaton D, Day M, Sutherland J, Heon E, Weleber RG, Gabriel LA, Cong P, Chuang K, Ye S, Sallum JM, Qi M. Exome sequencing identifies NMNAT1 mutations as a cause of Leber congenital amaurosis. Nat Genet. 2012;44(9):972–974. doi: 10.1038/ng.2370. [DOI] [PubMed] [Google Scholar]