Abstract
Neurofibromatosis type 1 is an autosomal dominant genetic disorder with an estimated birth incidence of 1 in 3000–4000. The major diagnostic criterion includes multiple cutaneous neurofibromas, axillary or inguinal freckling, and café au lait spots. Gastrointestinal neoplasms have a reported occurrence of 2–25 % of which neurofibromas are the most frequently diagnosed benign neoplasm. Periampullary tumors in patients with neurofibromatosis are usually carcinoids and very rarely adenocarcinoma. We report a case of 40-year old woman with neurofibromatosis type 1 who presented with epigastric pain and jaundice. She was diagnosed to have ampullary tumor after investigations, and she underwent pancreaticoduodenectomy. The resected specimen histologically showed adenocarcinoma of the ampulla.
Keywords: Neurofibromatosis type 1, Ampullary adenocarcinoma, Pancreaticoduodenectomy, Obstructive jaundice
Introduction
Neurofibromatosis type 1 is one of most common autosomal dominant inherited disorders affecting 1 in 3500 people. NF-1 gene has been identified on chromosome 17q11.2 [1]. Carriers of mutant NF-1 are predisposed to a variety of tumors including optic nerve glioma, neurofibroma and neurofibrosarcoma, malignant schwannomas, astrocytoma, and pheochromocytoma. The major diagnostic criterion includes multiple cutaneous neurofibromas, axillary or inguinal freckling, cafe au lait spots, Lisch nodules, (pigmented iris hamartoma), and the first-degree family history of NF-1 [2]. The ocular manifestation of NF-1 includes Lisch nodules, optic nerve glioma, and rarely conjunctival melanosis.
Gastrointestinal involvement is seen in 10–25 % of patients with NF-1 and causes symptoms in fewer than 5 % of patients [3]. The gastrointestinal manifestations of NF-1 occur in three forms:
Hyperplasia of the submucosal and myenteric nerve plexuses and mucosal ganglioneuromatosis, which typically occur in a patchy distribution and may appear as discrete neurofibromas or plexiform neurofibromas
Gastrointestinal stromal tumors that are located in the stomach and jejunum and are usually benign
A discrete glandular, somatostatin-rich carcinoid tumor of the periampullary region of the duodenum that contains psammoma bodies [4]
Gastrointestinal stromal tumors have been histologically identified as neurofibromas, leiomyomas, leiomyosarcomas, schwannomas, and stromal tumors without any definitive nerve or muscular differentiation [5]. The association of synchronous carcinoma of the ampulla of Vater and gastrointestinal stromal tumor of the jejunum has been rare.
We report a unique case of a patient with neurofibromatosis presenting with epigastric pain and obstructive jaundice due to ampullary adenocarcinoma.
Case Report
A 40-year-old woman affected with neurofibromatosis presented with a 2-month history of epigastric pain relieved by vomiting. There was no significant medical history. Family history included a daughter who is also affected by neurofibromatosis. On clinical examination, the patient had generalized neurofibromas. Ophthalmologic examination showed presence of Lisch nodules in both eyes and conjunctival melanosis in the right eye. The patient had icterus.
Laboratory examination showed mild hyperbilirubinemia (total bilirubin 1.8 mg %), mild elevation of alkaline phosphatase (210 IU/l). Gastroduodenoscopy showed a polypoid lesion at the ampulla and mucosa was intact. USG abdomen showed massive dilation of the biliary and pancreatic ducts. CT abdomen showed gross dilation of common bile duct (CBD) (3 cm) and pancreatic duct (2 cm) with intrahepatic biliary dilatation and grossly distended gall bladder. No evidence of peripancreatic, porta hepatic, or paraaortic lymph nodes was noted. Portal vein, superior mesenteric vein, and branches were normal. CT brain was normal and showed no optic nerve glioma or astrocytoma which these patients are predisposed to. With a diagnosis of the ampullary tumor with obstructive jaundice, a pancreaticoduodenectomy was planned. The patient was prepared for the surgery (Fig. 1).
Fig. 1.

Preoperative condition of the patient
An injection of vitamin K (10 mg) was given for three days preoperatively. The patient was started on intravenous antibiotics (ampicillin, amikacin, and ornidazole) and intravenous fluids 48 h prior to surgery. Bowel was prepared with polyethylene glycol. The abdomen was opened with a roof-top incision; operability was assessed, and liver and peritoneum were found to be normal with no deposits. A 2 cm tumor was palpated at the ampulla with no evidence of superior mesenteric vessel involvement. CBD was grossly dilated to 3 cm, and there was a very low insertion of cystic duct on posterior aspect of common hepatic duct, and pancreatic duct was dilated to more than 2 cm. There were nodules over the serosal surface of jejunum and a few lymph nodes. Pancreaticoduodenectomy with a Roux-en-Y loop end-to-side pancreaticojejunostomy, end-to-side hepaticojejunostomy, and antecolic gastrojejunostomy was performed (Fig. 2). No feeding jejunostomy was performed. However, a nasojejunal tube was kept intraoperatively. Postoperative period was uneventful. Oral feeds started on day 3. The patient was discharged on day 14. Histological analysis showed a polypoid growth measuring 2 cm in the region of ampulla, composed of cells arranged in glandular pattern, groups, cribiform pattern, and in linear cordlike fashion which show moderate nuclear pleomorphism, coarse granular chromatin, prominent nucleoli, and moderate amount of cytoplasm—all features suggestive of adenocarcinoma of ampulla of Vater (Fig. 3). The section from serosal nodules on the jejunum showed features of neurofibroma, and lymph nodes were free from tumor. Proximal and distal surgical margins were free from tumor.
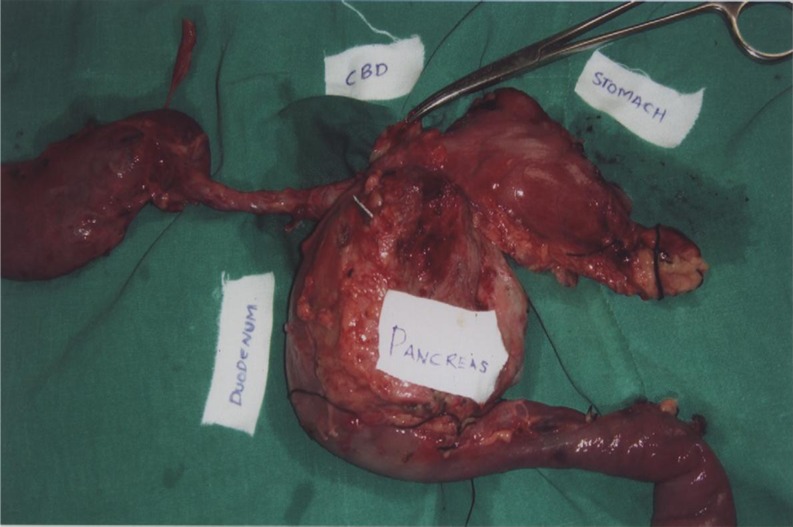
Fig. 2.
Pancreaticoduodenectomy specimen
Fig. 3.
Duodenal mucosa showing adenocarcinoma (1), Brunner gland (2), tumor tissue infiltrating the muscularis (3)
Discussion
Ampullary tumors are seen in patients with neurofibromatosis type 1 as a form of gastrointestinal manifestation. Periampullary tumors in patients with neurofibromatosis type 1 are usually carcinoids or stromal tumors and rarely adenocarcinomas [6]. A particular association has been noted between NF-1 and occurrence of ampullary carcinoid, with up to 25 % of these tumors being associated with NF-1 [7]. Although the stromal and carcinoid tumors are more commonly associated with neurofibromatosis, GI adenocarcinomas in NF-1 patients are reported in literature. They affect esophagus, stomach, duodenum, small bowel, colon, gallbladder, biliary tract, liver, and pancreas. In various studies in literature regarding tumors of periampullary region in NF-1 patients, three cases of adenocarcinoma were reported in addition to 19 cases of stromal tumors and 15 cases of carcinoid [6].
In our patient also, the occurrence of conjunctival melanosis in association with neurofibromatosis was seen which is considered to be a rare presentation in patients with neurofibromatosis.
Arnesjo hypothesized the presence of a multipotential neuroectodermal potentially evolving into both neural and endocrine cell lines in patients with neurofibromatosis type 1. According to various theories proposed by various authors, it is postulated that the simultaneous occurrence of stromal tumors and or carcinoids and epithelial tumors may be due to the presence of multipotential cell lines which could differentiate into above said cell lines [8].
In accordance with these theories, the risk of neoplastic degeneration of nonneoplastic tissue adjacent to the known ampullary or periampullary neoplasm, a widely demolative surgery like pancreaticoduodenectomy is the surgical procedure of choice.
Conclusion
In conclusion, our case represents a rare presentation of adenocarcinoma of ampulla of Vater in a patient with neurofibromatosis type 1 and also occurrence of small bowel neurofibromas. The report also emphasizes the fact that these patients need demolative surgery in the form of pancreaticoduodenectomy.
Also, these patients are predisposed to occurrence of conjunctival melanosis which in long run can turn to malignancy.
References
- 1.Barker D, Wright E, Nguyen K, Cannon L, Fain P, Goldgar D, et al. Gene for Von Recklinghausens neurofibromatosis is in the pericentromeric region of chromosome 17. Science. 1987;236:1100–1102. doi: 10.1126/science.3107130. [DOI] [PubMed] [Google Scholar]
- 2.Riccardi VM. Von Recklinghausen neurofibromatosis. N Engl J Med. 1981;305:1617–1627. doi: 10.1056/NEJM198112313052704. [DOI] [PubMed] [Google Scholar]
- 3.Seymour-Dempsey K, Andrassy RJ. Neurofibromatosis implications for the general surgeon. J Am Coll Surg. 2002;195:553–563. doi: 10.1016/S1072-7515(02)01252-8. [DOI] [PubMed] [Google Scholar]
- 4.Behranwala KA, Spalding D, Wotherspoon A, Fischer C. Small bowel GIST and ampullary cancer in Type1 neurofibromatosis. World J Surg Oncol. 2004;2:1. doi: 10.1186/1477-7819-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller CE, Williams GT. Gastrointestinal manifestations of type 1 neurofibromatosis (Von Recklinghausens disease) Histopathology. 1991;19:1–11. doi: 10.1111/j.1365-2559.1991.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 6.Costi R, Caruana P, Sarli L, Violi V, Roncorni L, et al. Ampullary adenocarcinoma in neurofibromatosis type 1. Case report. Mod Pathol. 2001;14(11):1169–1174. doi: 10.1038/modpathol.3880454. [DOI] [PubMed] [Google Scholar]
- 7.Hatzitheoklitos E, Buchler MW, Friess H, et al. Carcinoid of the ampulla of Vater. Clinical characteristics and morphologic features. Cancer. 1994;73:1580–1588. doi: 10.1002/1097-0142(19940315)73:6<1580::AID-CNCR2820730608>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Arnesjo B, Idvoll I, Ishe I, Telenivs M, Tylen U. Concomitant occurrence of neurofibromatosis and carcinoid of the intestine. Scand J Gastroenterol. 1973;8:637–643. [PubMed] [Google Scholar]