Figure 1.
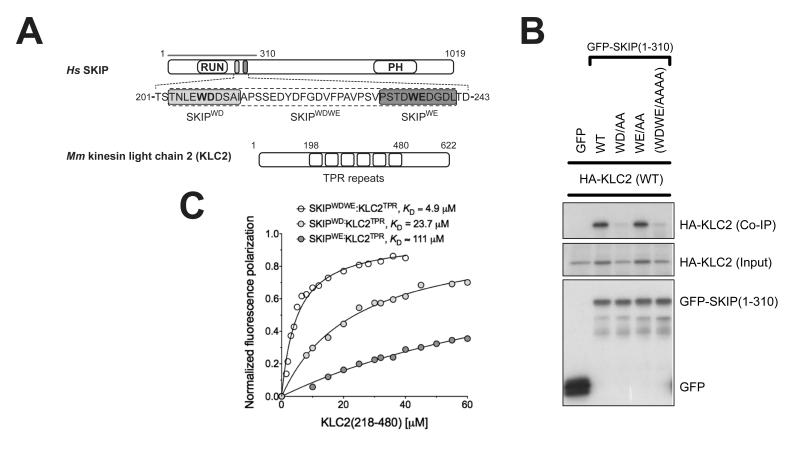
Binding of SifA-kinesin binding protein (SKIP) to kinesin light chain 2 (KLC2). (A) Scheme of SKIP and KLC2. The two W-acidic motifs and KLC2 TPR repeats are highlighted. (B) Co-immunoprecipitation shows that alanine replacement in the first W-acidic motif (WD) abrogates SKIP:KLC2 binding whereas the same substitution in the second motif (WE) has virtually no effect. (C) Fluorescence polarization measurements confirm that the first SKIP W-acidic motif (SKIPWD) has a higher affinity for the TPR domain of KLC2 than the second one (SKIPWE). A peptide encompassing both motifs (SKIPWDWE) binds with higher affinity than the best single motif. KD values reported here were calculated at 150 mM NaCl. Binding affinity values at varying ionic strength values are given in Fig. S1.