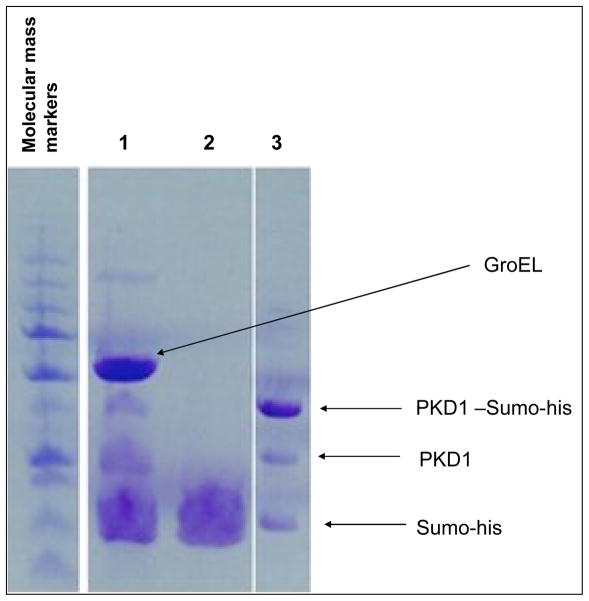
Figure 9.
Sumo tag is from the mPKD11–193 –Sumo-his can be removed even in the presence of GroEL. The partially folded polycystin-1 remains bound to GroEL (no evidence of aggregation).
Lane 1 – Retentate on Microcon YM-10 before addition of ATP (final concentration 5 mM): GroEL-PKD1- -complex, treated with SUMO-protease (~31kDa)(sumolyase): GroEL (~60 kDa); mPKD11–193 –SUMO (~37 kDa); mPKD11–193 –(~24 kDa); SUMO-tag (~12 kDa);
Lane 2 – Filtrate through Microcon YM-10 after addition of ATP: SUMO-tag (~12 kDa) was the only protein observed in the filtrate;
Lane 3 – In a separate experiment mPKD11–193 SUMO partially cleaved with SUMO-protease: mPKD11–193 –SUMO(~37 kDa)- (PKD1-SUMO-his); mPKD11–193 –(~24 kDa) (PKD1); SUMO-tag (~12 kDa) (SUMO-His). This latter experiment provides reference molecular mass markers for the experiments with GroEL, mPKD11–193 – Sumo-his and all of the cleavage products.