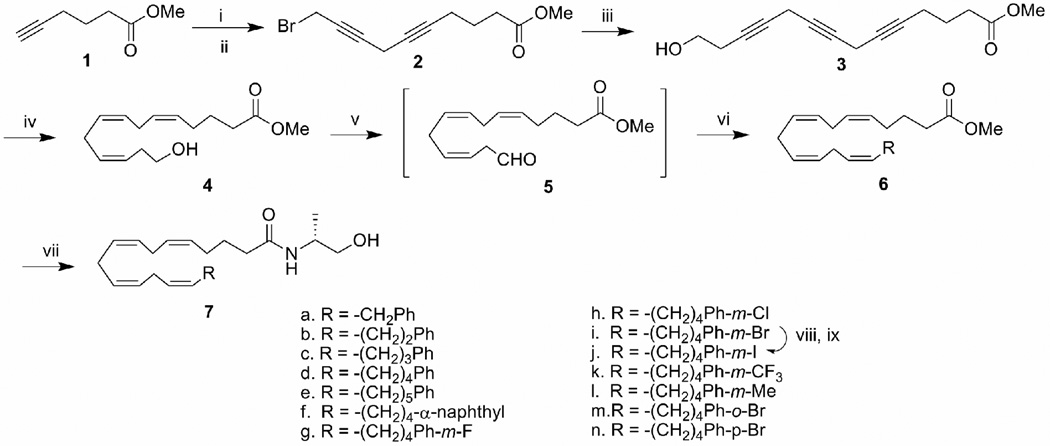
Scheme 1.
Reagents and conditions: (i). 4-chloro-butyn-1-ol, CuI, NaI, K2CO3, DMF, rt, overnight, 84%; (ii). PPh3, CBr4, rt, 95%; (iii). 3-butyn-1-ol, CuI, NaI, K2CO3, DMF, rt, overnight, 80%; (iv). H2, Lindlar catalyst, quinoline, ether, 0–10 °C, 86%; (v). Dess-Martin reagent, CH2Cl2, rt, 30 min, 86%; (vi). Wittig reagent from the corresponding phosphonium salt (see scheme 2) with n-BuLi in THF at −78° C, 60–80%;(vii). R-(−)-2-amino-propanol, NaCN (cat), Methanol, 55°C, sealed vial, 74%; (viii). bis-tributyltin, Pd(PPh3)4, toluene, reflux, 40%; (ix). I2, CH2Cl2, rt, 95%.