Table 1.
Affinity (Ki)a of new anandamide analogues for CB1 and CB2 receptors
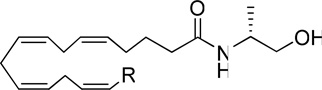 | |||
|---|---|---|---|
| Analogues | R | CB1 Ki (nM) (PMSF) |
CB2 Ki (nM) |
| (R)-methanandamide | (CH2)4CH3 | 17.9 | 868 |
| 7a | CH2Ph | 348.5 | 826.3 |
| 7b | (CH2)2Ph | 241.2 | 335 |
| 7c | (CH2)3Ph | 69.2 | 533.7 |
| 7d | (CH2)4Ph | 13.3 | 1117 |
| 7e | (CH2)5Ph | 76.6 | 240.6 |
| 7f | (CH2)4-α-naphthyl | 678.3 | 3996 |
| 7g | (CH2)4Ph-m-F | 30.4 | 987.3 |
| 7h | (CH2)4Ph-m-Cl | 89.4 | 605.1 |
| 7i | (CH2)4Ph-m-Br | 95.3 | 1054 |
| 7j | (CH2)4Ph-m-I | 533.3 | 1564 |
| 7l | (CH2)4Ph-m-Me | 344.3 | 839.7 |
| 7m | (CH2)4Ph-o-Br | 170.0 | N.R. |
| 7n | (CH2)4Ph-p-Br | 8.9 | 250.4 |
| 7o | (CH2)4(3-furyl) | 12.0 | 1027 |
| 7p | (CH2)5(3-furyl) | 170 | 869 |
| 7q | (CH2)4(2-furyl) | 325 | 749.8 |
| 7s | (CH2)5(2-furyl) | 561.2 | 3255 |
CB1 affinities were determined using rat brain membranes and 0.8 nM [3H] CP-55,940 as the radioligand. Mouse spleen was used as source of CB2 receptor. Data were analyzed using nonlinear regression analysis. Ki values were obtained from a minimum of two independent experiments run in duplicate.
