Abstract
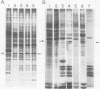
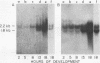
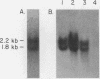
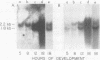
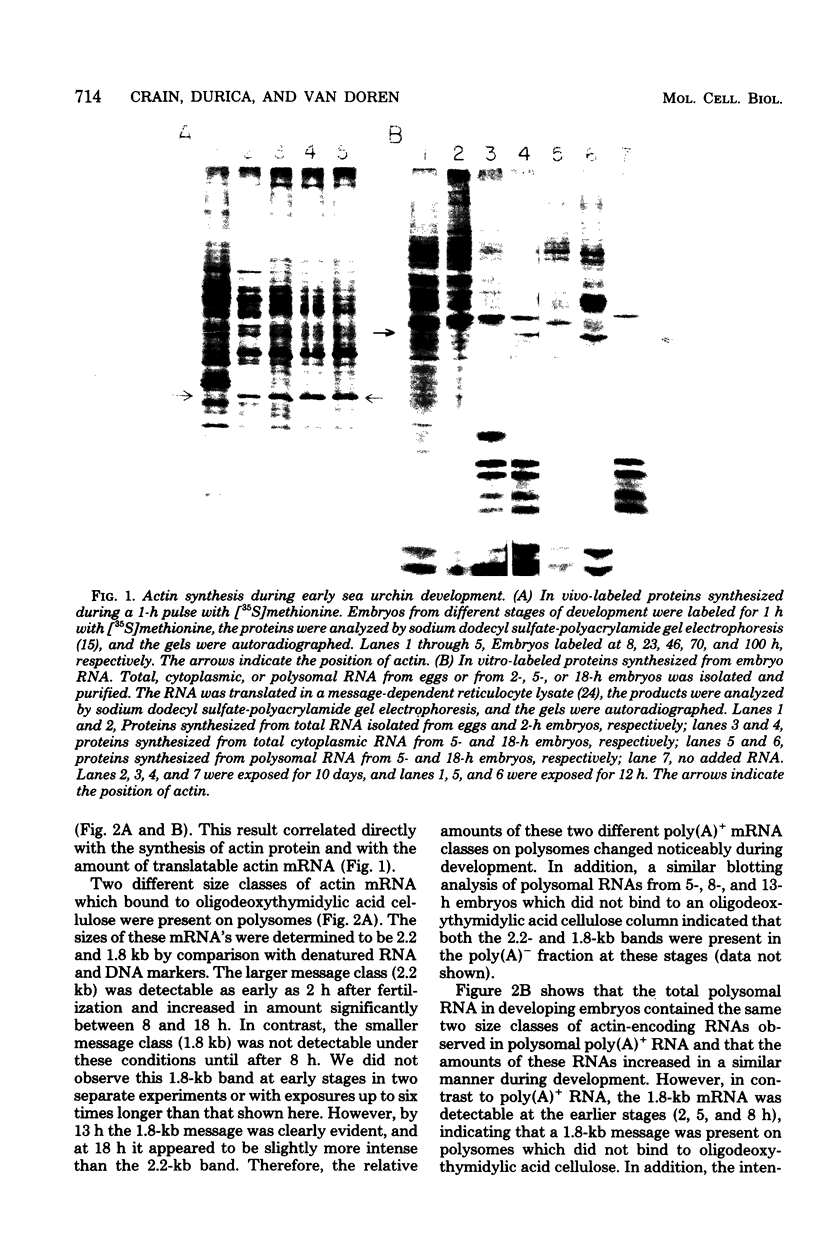
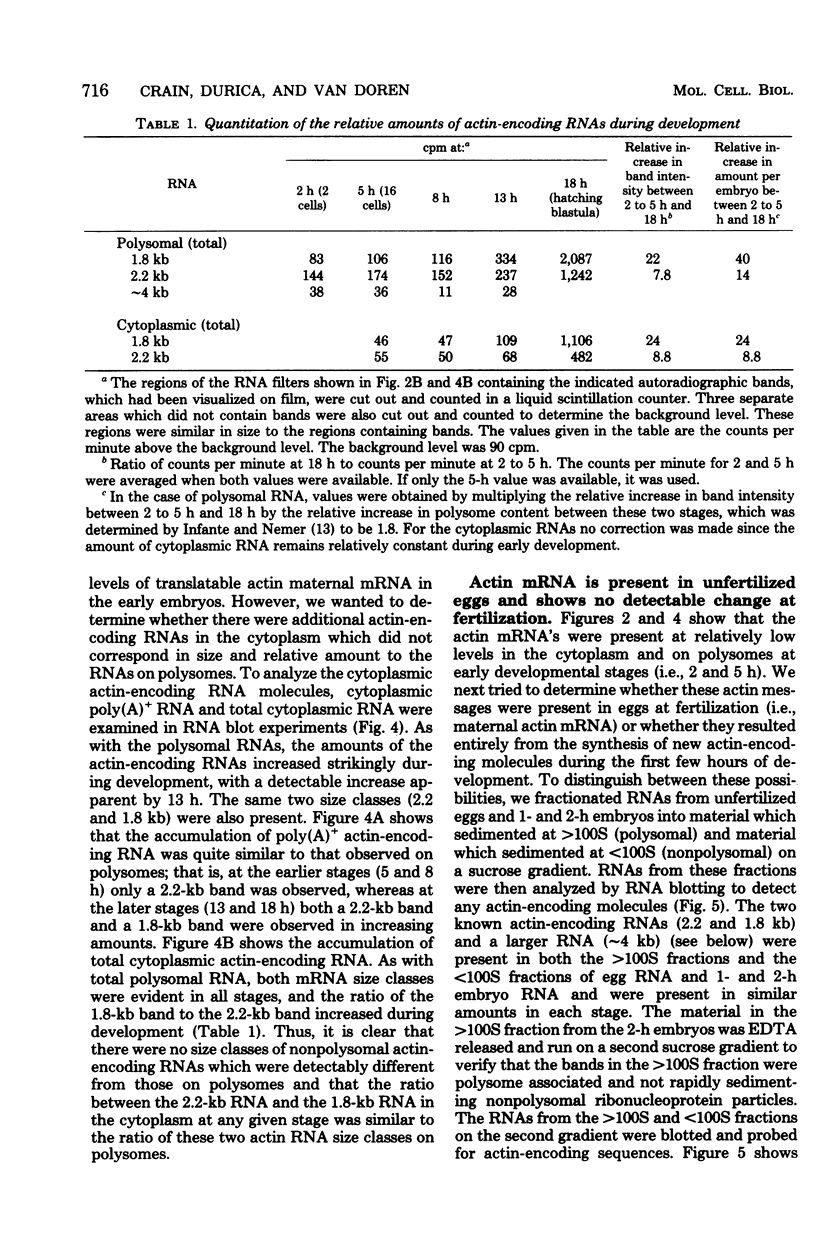
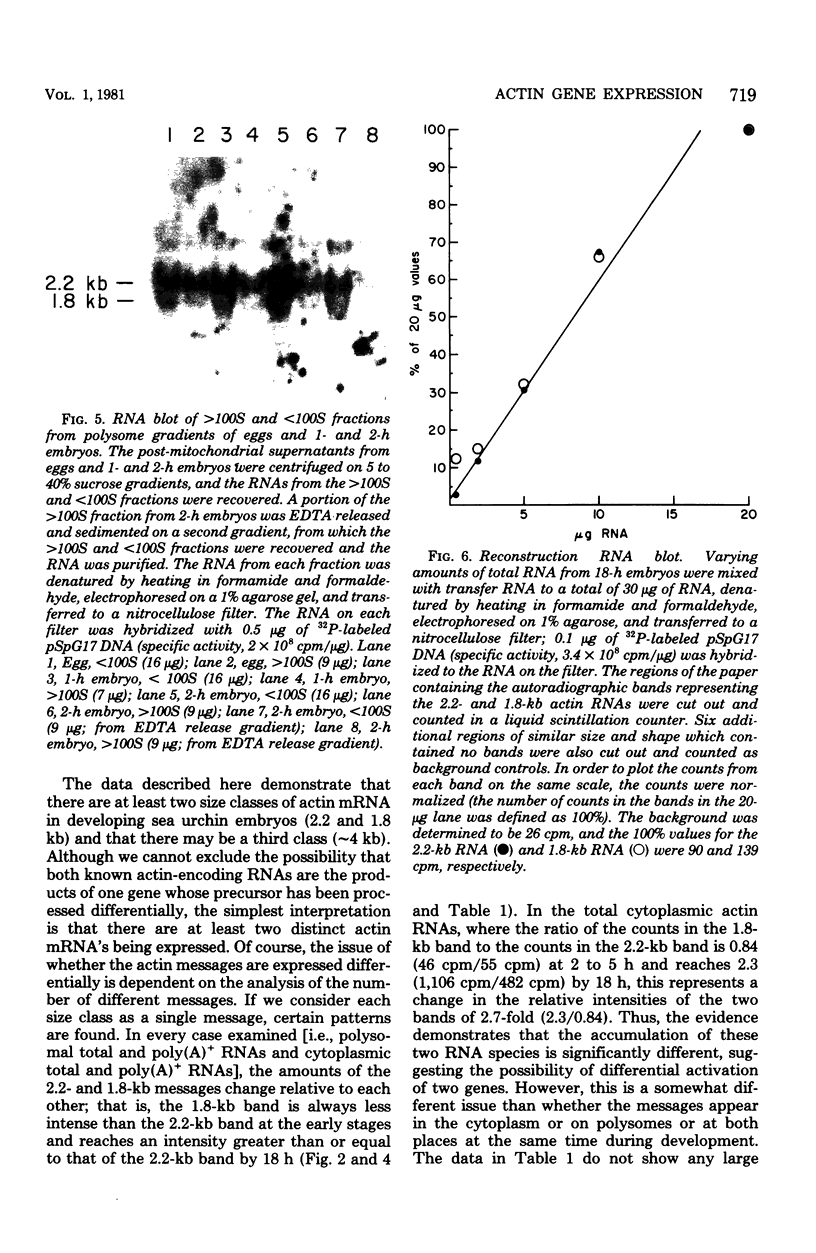
We show that the synthesis of actin is regulated developmentally during early sea urchin embryogenesis and that the level of synthesis of this protein parallels the steady-state amounts of the actin messenger ribonucleic acids (RNA). An in vitro translation and RNA blotting analysis of embryo RNA from several stages of early development indicated that during the first 8 h after fertilization there was a low and relatively constant level of actin messenger RNA in the embryo. Between 8 and 13 h of development, the amount of actin messenger RNA began to increase both in the cytoplasm and on polysomes, and by 18 h the amounts of actin message per embryo had risen between approximately 10- and 25-fold in the cytoplasm and between 15- and 40-fold on polysomes. Two size classes of actin messenger RNA (2.2 and 1.8 kilobases) were identified in unfertilized eggs and in all of the developmental stages examined. The amount of each actin message class increased over a similar time interval during early development. However, the amounts of these size classes in the cytoplasm relative to each other shifted between the earliest stages examined (2 to 5 h) and the hatching blastula stage (18 h), with the ratio of the 1.8-kilobase actin messenger RNA to the 2.2-kilobase actin messenger RNA increasing almost threefold during this period.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S. L., Birkenmeier C. S. Inhibition of intractable nucleases with ribonucleoside--vanadyl complexes: isolation of messenger ribonucleic acid from resting lymphocytes. Biochemistry. 1979 Nov 13;18(23):5143–5149. doi: 10.1021/bi00590a018. [DOI] [PubMed] [Google Scholar]
- Brandhorst B. P., Humphreys T. Stabilities of nuclear and messenger RNA molecules in sea urchin embryos. J Cell Biol. 1972 May;53(2):474–482. doi: 10.1083/jcb.53.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Durica D. S., Schloss J. A., Crain W. R., Jr Organization of actin gene sequences in the sea urchin: molecular cloning of an intron-containing DNA sequence coding for a cytoplasmic actin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5683–5687. doi: 10.1073/pnas.77.10.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P. R. The control of protein synthesis in embryonic development and differentiation. Curr Top Dev Biol. 1967;2:1–46. doi: 10.1016/s0070-2153(08)60282-3. [DOI] [PubMed] [Google Scholar]
- Hough-Evans B. R., Wold B. J., Ernst S. G., Britten R. J., Davidson E. H. Appearance and persistence of maternal RNA sequences in sea urchin development. Dev Biol. 1977 Oct 1;60(1):258–277. doi: 10.1016/0012-1606(77)90123-3. [DOI] [PubMed] [Google Scholar]
- Humphreys T. Measurements of messenger RNA entering polysomes upon fertilization of sea urchin eggs. Dev Biol. 1971 Oct;26(2):201–208. doi: 10.1016/0012-1606(71)90122-9. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Nemer M. Accumulation of newly synthesized RNA templates in a unique class of polyribosomes during embryogenesis. Proc Natl Acad Sci U S A. 1967 Aug;58(2):681–688. doi: 10.1073/pnas.58.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedes L. H., Gross P. R. Synthesis and function of messenger RNA during early embryonic development. J Mol Biol. 1969 Jun 28;42(3):559–575. doi: 10.1016/0022-2836(69)90243-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Lev Z., Xin J. H., Britten R. J., Davidson E. H. Messenger RNA prevalence in sea urchin embryos measured with cloned cDNAs. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5317–5321. doi: 10.1073/pnas.77.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev Z., Thomas T. L., Lee A. S., Angerer R. C., Britten R. J., Davidson E. H. Developmental expression of two cloned sequences coding for rare sea urchin embryo messages. Dev Biol. 1980 May;76(2):322–340. doi: 10.1016/0012-1606(80)90382-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Merlino G. T., Water R. D., Chamberlain J. P., Jackson D. A., El-Gewely M. R., Kleinsmith L. J. Cloning of sea urchin actin gene sequences for use in studying the regulation of actin gene transcription. Proc Natl Acad Sci U S A. 1980 Feb;77(2):765–769. doi: 10.1073/pnas.77.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer M., Dubroff L. M., Graham M. Properties of sea urchin embryo messenger RNA containing and lacking poly(A). Cell. 1975 Oct;6(2):171–178. doi: 10.1016/0092-8674(75)90007-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Shepherd G. W., Nemer M. Developmental shifts in frequency distribution of polysomal mRNA and their posttranscriptional regulation in the sea urchin embryo. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4653–4656. doi: 10.1073/pnas.77.8.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]