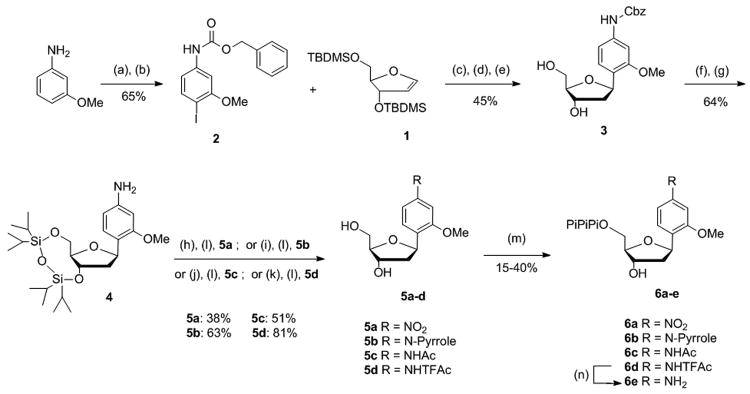
Scheme 1. Conditions: (a) CBz-Cl, NaHCO3, THF, rt, 20 min; (b) I2, Ag2SO4, MeOH, - 20 °C, 1 h; (c) Pd(OAc)2, AsPh3, nBu3N, DMF, 70 °C, 15 h; (d) TBAF 1 m in THF, 0 °C → rt, 2 h; (e) NaBH(OAc)3, AcOH, CH3CN, 0 °C, 1 h; (f) 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane, pyridine, rt, 2 h; (g) 10% Pd/C, H2, EtOAc, rt, 1 h; (h) KI, tbutyl hydroperoxide aq 70%, CH3CN, dark, 75 °C, 2 h; (i) 2,5-dimethoxytetrahydrofuran, H2O, microwave-140 °C, 30 min; (j) Ac2O, Et3N, CH2Cl2, rt, 20 min; (k) trifluoroacetic anhydride, Et3N, CH2Cl2, 10 °C, 20 min; (l) TBAF 1 m in THF, 1 h; (m) proton sponge, POCl3, PO(OMe)3, -15 °C → -10 °C, 3 h then Bu3N, (Bu3NH)2H2P2O7 in DMF, -10 °C → 0 °C, 30 min then TEAB buffer (0.5 m), rt, 10 min; (n) NH4OH 30%, rt, 1 h.
