Abstract
Background
Refractory pseudophakic cystoid macular edema (PCME) following cataract surgery has long posed a challenge to clinicians, but intravitreal injections with a sustained delivery 0.7 mg dexamethasone implant has emerged as a promising therapy for this condition.
Objective
To present a case of longstanding and refractory PCME with complete remission through 189 days of follow-up after two successive injections with intravitreal dexamethasone implants.
Case report
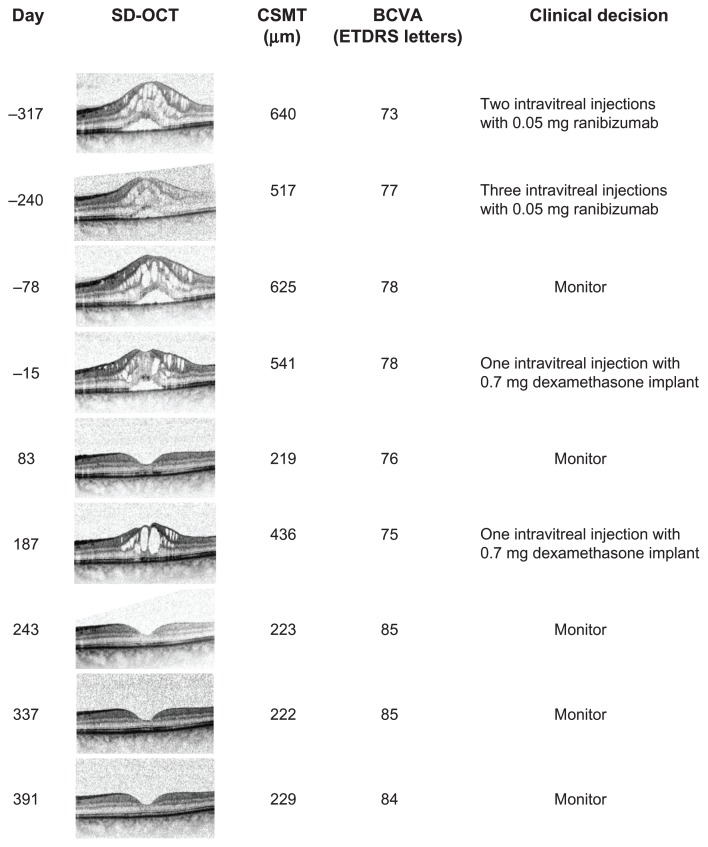
A 59-year-old male had experienced metamorphopsia for approximately 4 years and had been diagnosed with PCME 15 months earlier. Since the time of the diagnosis, the condition had been refractory to both subtenon triamcinolone acetonide and a total of five injections with intravitreal ranibizumab. After the last injection with ranibizumab, central subfield mean thickness was 640 μm, and the best corrected visual acuity was 78 Early Treatment Diabetic Retinopathy Study letters. Following an intravitreal injection with a dexamethasone implant, the macular edema resolved at the next follow-up. The macular edema returned 187 days after the first injection and was treated with another intravitreal dexamethasone implant. Again, the macular edema subsided completely, and best corrected visual acuity improved to 84 Early Treatment Diabetic Retinopathy Study letters, a condition which was maintained through an additional 189 days of follow-up.
Conclusion
Chronic PCME is traditionally a difficult condition to treat, but we are encouraged by the optimal response experienced with intravitreal sustained release dexamethasone implants in our patient whose longstanding PCME had been refractory to previous treatments with both subtenon triamcinolone and intravitreal ranibizumab. In this case, the condition appeared to be fully reversible once inflammation was controlled, but the need for monitoring and repeated injections remains an issue of concern.
Keywords: intravitreal dexamethasone implant, pseudophakic cystoid macular edema, Ozurdex®, Irvine-Gass syndrome
Introduction
Cystoid macular edema occurs in many retinal disorders including diabetic maculopathy, retinal vein occlusion, uveitis, and pseudophakic cystoid macular edema (PCME) following cataract surgery. It is generally accepted that inflammation plays a dominant role in PCME.1 When refractory to topical nonsteroidal anti-inflammatory drugs or topical corticosteroids, these conditions have long posed a challenge to clinicians as available therapies have yielded mixed results.1 In PCME, intravitreal corticosteroids have the advantage of being a potent anti-inflammatory agent, while there is no need to consider the common side effect of cataract formation. Recently, sustained delivery 0.7 mg dexamethasone implants (Ozurdex®, Allergan, Irvine, CA, USA) have been introduced for ocular use. Some authors have suggested that intravitreal dexamethasone implants should be considered first-line therapy in chronic PCME,2 though intravitreal triamcinolone, ranibizumab, and bevacizumab have also been shown to be effective in some, but not all, reports.1
We present a case of longstanding PCME that had not responded to subtenon triamcinolone acetonide, or intravitreal injections with ranibizumab but improved significantly following intravitreal injections with a dexamethasone implant.
Case report
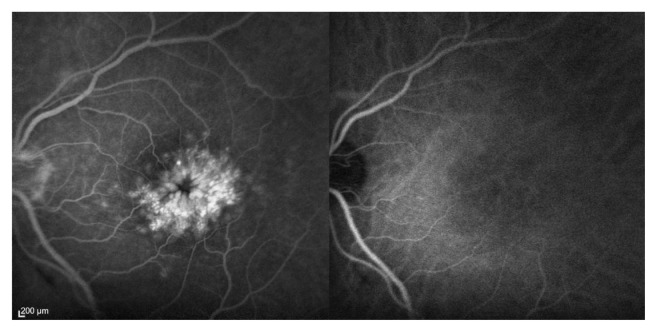
A 59-year-old male presented to an ophthalmology department with a 2.5-year history of unilateral metamorphopsia. The patient was healthy, did not have diabetes, and had no ophthalmological history apart from uneventful cataract surgery approximately 6 months prior to the first experience of metamorphopsia. Funduscopy, fluorescein angiography (SPECTRALIS® OCT, Heidelberg Engineering, Heidelberg, Germany) (Figure 1) and spectral domain optical coherence tomography (OCT) (SPECTRALIS® OCT) were performed, and PCME with an underlying serous macular detachment was diagnosed. There was no sign of choroidal neovascularizations, and there were no signs of inflammation. The best corrected visual acuity (BCVA) was 0.5 on the Snellen chart, corresponding to approximately 70 Early Treatment Diabetic Retinopathy Study (ETDRS) letters. The fellow eye was normal. After 2 months, a subtenon injection with corticosteroid (40 mg triamcinolone acetonide) was administered, but the macular edema and BCVA was unaffected through 2 months of follow-up. The patient was referred to our department, where the central subfield mean thickness on OCT measured 640 μm, and BCVA was 73 letters on the ETDRS visual chart (Figure 2). In the following 11 months, a total of five intravitreal injections with off-label anti-vascular endothelial growth factor (0.05 mg ranibizumab Lucentis®; Novartis, Basel, Switzerland) were performed. The macular edema remained, with a central subfield mean thickness of 625 μm on OCT, but BCVA improved somewhat to 78 ETDRS letters (Figure 2). Dexamethasone implants had now become available for ocular use at our department, and we performed an off-label intravitreal injection, with a sustained release 0.7 mg dexamethasone implant. The patient gave informed consent and was aware of the off-label nature of the treatment. Eighty-three days postinjection, the macular edema had subsided completely with a central subfield mean thickness of 219 μm, though BCVA remained 76 ETDRS letters. The macular edema recurred 187 days postinjection, and another dexamethasone implant injection was performed. The edema subsided, and BCVA improved to 85 ETDRS letters and was maintained for an additional 189 days of follow-up, after the second injection (Figure 2).
Figure 1.
Fluorescein (left) and indocyanine green (right) angiography 122 seconds after dye injection. This angiography was performed 11 months prior to the first intravitreal dexamethasone implant injection.
Figure 2.
Progression of pseudophakic cystoid macular edema and visual acuity following intravitreal injection with ranibizumab and dexamethasone implants.
Notes: All images were aligned during recording using eye tracking software. Day refers to days before or after the first dexamethasone implant injection.
Abbreviations: BCVA, best corrected visual acuity; CSMT, central subfield mean thickness; ETDRS, Early Treatment Diabetic Retinopathy Study; SD-OCT, spectral domain optical coherence tomography.
Discussion
The slow onset of symptoms in this patient is somewhat uncommon in PCME, but the prior cataract surgery makes this diagnosis the most likely. An alternative diagnosis could be idiopathic macular edema, but inflammation would still be the most probable underlying pathogenesis. Dexamethasone has a 6-fold more potent anti-inflammatory profile than triamcinolone acetonide,2 and subtenon application leads to a lower intravitreal concentration compared to intravitreal sustained delivery.3 This could explain the lack of response following the subtenon application of triamcinolone acetonide in our patient. Previous reports of the treatment of refractory PCME with intravitreal anti-vascular endothelial growth factor have been small, focused on bevacizumab, and have reported mixed findings.4–7 Intravitreal triamcinolone injection was not considered for this patient due to efficacy8 and safety9 concerns. Our patient was never treated with topical steroids or nonsteroidal anti-inflammatory drugs.
The Dexamethasone DDS Phase II study group did a subgroup analysis of patients with uveitic macular edema or PCME combined.10 They reported visual gain at 90 days of follow-up of at least 15 EDTRS letters in one of 14 patients in the observational group, two of 12 patients treated with a 0.35 mg intravitreal dexamethasone implant, and seven of 13 patients treated with a 0.7 mg intravitreal dexamethasone implant. The study did not report on duration of symptoms, measurements of macular edema, or retreatment algorithm. A case report presented a patient that had not responded to an intravitreal 0.4 mg nonimplant dexamethasone injection, but had complete remission of macular edema and an improved BCVA, from 0.3 to 0.8 Snellen equivalents, following a 0.7 mg dexamethasone implant.11 These reports are consistent with our findings; however, to our knowledge, the present case is the first description of a treatment-refractory PCME, lasting at least 15 months, that was subsequently successfully treated with sustained release intravitreal 0.7 mg dexamethasone implants.
Conclusion
Chronic PCME is traditionally a difficult condition to treat, but we are encouraged by the optimal response observed with intravitreal sustained release dexamethasone implants in our patient who had previously shown no improvement after treatment with both subtenon triamcinolone and intravitreal ranibizumab for a longstanding PCME. In this case, the condition appeared to be fully reversible once inflammation was controlled, but the need for monitoring and repeated injections remains an issue of concern.
Footnotes
Disclosure
TLS is an advisory board member of Allergan. The other authors report no conflicts of interest in this work.
References
- 1.Arevalo JF, Maia M, Garcia-Amaris RA, et al. Pan-American Collaborative Retina Study Group (PACORES) Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: the Pan-American Collaborative Retina Study Group results. Ophthalmology. 2009;116:1481–1487. doi: 10.1016/j.ophtha.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Herrero-Vanrell R, Cardillo JA, Kuppermann BD. Clinical applications of the sustained-release dexamethasone implant for treatment of macular edema. Clin Ophthalmol. 2011;5:139–146. doi: 10.2147/OPTH.S15783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue M, Takeda K, Morita K, Yamada M, Tanigawara Y, Oguchi Y. Vitreous concentrations of triamcinolone acetonide in human eyes after intravitreal or subtenon injection. Am J Ophthalmol. 2004;138(6):1046–1048. doi: 10.1016/j.ajo.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Barone A, Russo V, et al. Short-term safety and efficacy of intravitreal bevacizumab for pseudophakic cystoid macular edema. Retina. 2009;29(1):33–37. doi: 10.1097/IAE.0b013e31818a1fbc. [DOI] [PubMed] [Google Scholar]
- 5.Demirel S, Batiolu F, Özmert E. Intravitreal ranibizumab for the treatment of cystoid macular edema in Irvine-Gass syndrome. J Ocul Pharmacol Ther. 2012;28(6):636–639. doi: 10.1089/jop.2012.0032. [DOI] [PubMed] [Google Scholar]
- 6.Mason JO, III, Albert MA, Jr, Vail R. Intravitreal bevacizumab (Avastin) for refractory pseudophakic cystoid macular edema. Retina. 2006;26:356–357. doi: 10.1097/00006982-200603000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Spitzer MS, Ziemssen F, Yoeruek, et al. Efficacy of intravitreal bevacizumab in treating postoperative pseudophakic cystoid macular edema. J Cataract Refract Surg. 2008;34(1):70–75. doi: 10.1016/j.jcrs.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Sørensen TL, Haamann P, Villumsen J, Larsen M. Intravitreal triamcinolone for macular oedema: efficacy in relation to aetiology. Acta Ophthalmol Scand. 2004;83(1):67–70. doi: 10.1111/j.1600-0420.2004.00336.x. [DOI] [PubMed] [Google Scholar]
- 9.Kai W, Yanrong J, Xiaoxin L. Vehicle of triamcinolone acetonide is associated with retinal toxicity and transient increase of lens density. Graefes Arch Clin Exp Ophthalmol. 2006;244(9):1152–1159. doi: 10.1007/s00417-005-0251-9. [DOI] [PubMed] [Google Scholar]
- 10.Williams GA, Haller JA, Kuppermann BD, et al. Dexamethasone DDS Phase II Study Group. Dexamethasone posterior-segment drug delivery system in the treatment of macular edema resulting from uveitis or Irvine-Gass syndrome. Am J Ophthalmol. 2009;147(6):1048–1054. doi: 10.1016/j.ajo.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 11.Meyer LM, Schönfeld CL. Cystoid macular edema after complicated cataract surgery resolved by an intravitreal dexamethasone 0.7-mg implant. Case Rep Ophthalmol. 2011;2(3):319–322. doi: 10.1159/000332424. [DOI] [PMC free article] [PubMed] [Google Scholar]