Figure 1.
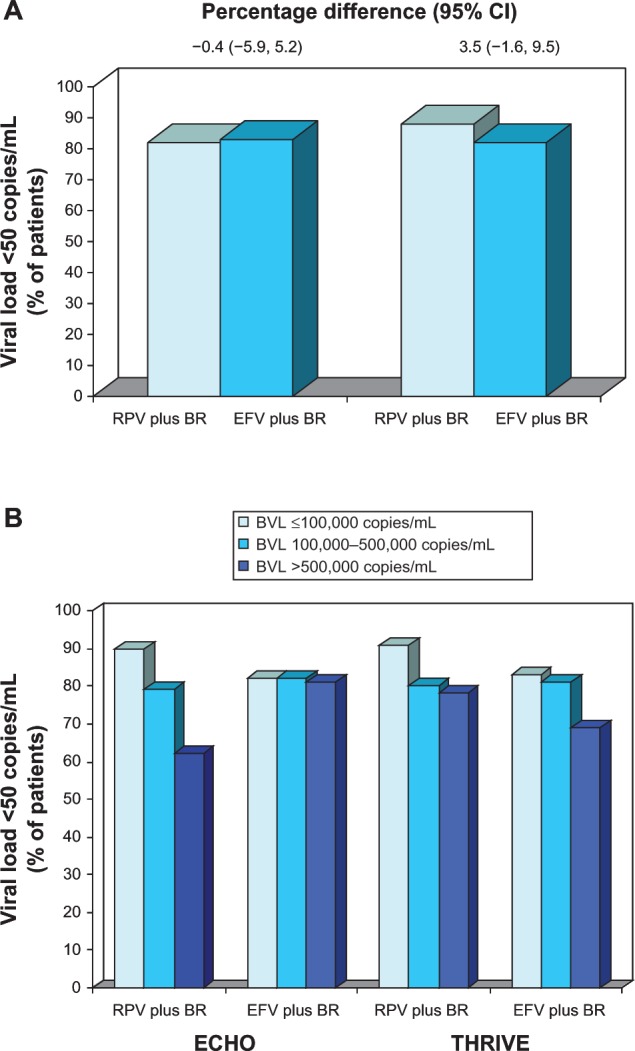
Efficacy of oral rilpivirine as a component of combination therapy in antiretroviral-naïve patients with HIV infection.
Notes: Response rates (A) in the full modified intent-to-treat populations (primary end point) and (B) in descriptive subgroup analyses according to baseline HIV-RNA level. Results are from the randomized, double-blind, double-dummy, multinational ECHO (n = 690)24 and THRIVE (n = 678)25 trials, which compared rilpivirine 25 mg once daily with efavirenz 600 mg once daily, plus background regimens. For the primary analysis, the noninferiority margin (rilpivirine vs efavirenz) for the lower bound of the 95% CI was −12%. In the ECHO and THRIVE trials, 344 and 354, 265 and 254, and 81 and 70 patients, respectively, had baseline HIV-RNA levels of ≤100,000, 100,000–500,000, and >500,000 copies/mL.
Abbreviations: BR, background regimen; BVL, baseline viral load; CI, confidence interval; EFV, efavirenz; RPV, rilpivirine; RNA, ribonucleic acid.
