Figure 2.
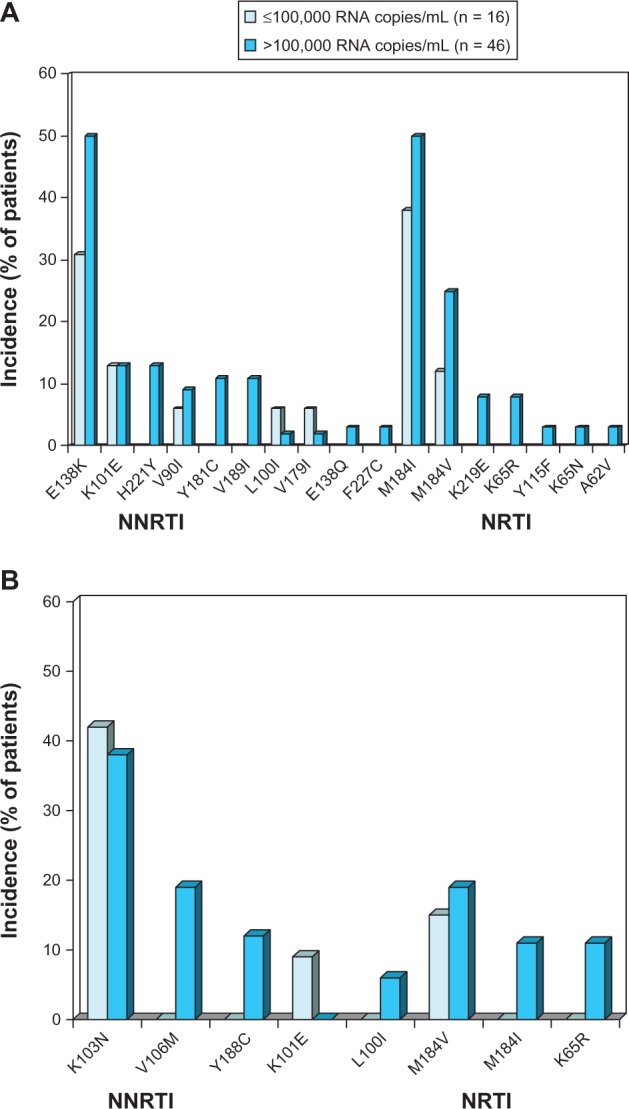
Resistance-associated mutations occurring in ≥2 patients at time of virological failure with (A) once-daily oral rilpivirine 25 mg plus a background regimen or (B) efavirenz 600 mg plus a background regimen.
Notes: Data are from a pooled analysis26 of the Phase III ECHO24 and THRIVE25 trials. Patients with evaluable post-baseline resistance data at 48 weeks were included and the data are presented according to baseline HIV viral load.
Abbreviations: NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide-reverse transcriptase inhibitor; RNA, ribonucleic acid.
