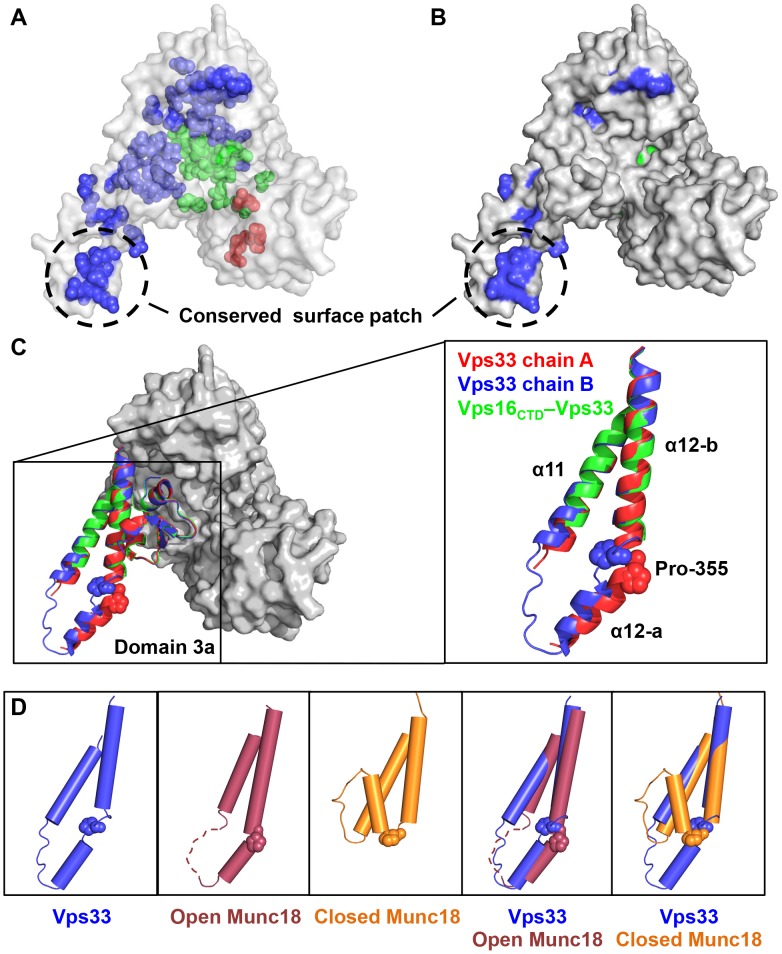
Figure 6. Domain 3a displays an open conformation featuring conserved surface-exposed residues.
(A) Highly conserved residues were determined by comparing the sequences of fifteen Vps33 orthologs from yeast to human and are shown on the C. thermophilum structure as spheres. (B) A surface representation reveals that a majority of the conserved surface-exposed residues map to domain 3a. Except in domain 3a, few surface-exposed conserved residues are visible on the ‘back’ side of Vps33 (not shown). (C) The two Vps33 monomers present in the asymmetric unit (chains A and B), while highly similar overall, show significant structural divergence in domain 3a. Pro-355, a potential hinge residue [14], is highlighted. The tip of loop 3a was not visible in the Vps16CTD–Vps33 complex. (D) Superposition with open and closed Munc18 structures reveals that Vps33 domain 3a adopts an open conformation. Also shown are the relevant regions of open rat Munc18–1 (PDB entry 3PUJ, which includes the N-peptide of syntaxin 4) and closed M. brevicollis Munc18 (2XHE, which includes syntaxin 1).