Abstract
Background
The relationship between obesity, physical fitness, and inflammation was analyzed in a Polish population aged 12 to 18 years.
Material/Methods
Body mass index measurements and Eurofit physical fitness tests were undertaken to assess the adiposity and physical fitness status, respectively, of subjects. Serum samples were collected to measure standard inflammatory markers, including interleukin 6; and the acute-phase proteins alpha1-acid glycoprotein and alpha1-antichymotrypsin. In addition, the glycosylation profiles of alpha1-acid glycoprotein and alpha1-antichymotrypsin were analyzed to further evaluate immune statuses.
Results
The physical fitness of individuals was negatively influenced by obesity. Obese subjects were characterized by an abnormal immune balance, including increased levels of alpha1-acid glycoprotein, as well as alpha1-antichymotrypsin, and altered glycosylation profiles indicative of an underlying inflammatory condition. Older age, male sex, and a large body mass index appeared to correlate with poor physical fitness scores and a disturbed immune status.
Conclusions
Impaired physical fitness is indicative for non-compensated overweight and affects mainly males aged 15 to 18 years. Female subjects seemed to cope better with increased body mass.
Keywords: adolescents, inflammation, obesity, physical fitness
Background
Childhood obesity is a growing epidemic. The prevalence of overweight and obesity in children is increasing dramatically worldwide [1,2]. Since the 1960s, the proportion of overweight adolescents globally has increased. Approximately 15% of all 15- year-olds in the United States have been reported to be obese [3]. Similarly, in the last 20 years, a dramatic increase in the proportion of overweight and obese children in the European Union (EU) has taken place [4]. Approximately 30% of European children are overweight and approximately one-quarter of them have been reported to be obese [4].
In Poland, between the years 1994 and 1995, a population-based study was conducted that assessed the prevalence of overweight and obesity amongst children between 6 and 17 years of age [5]. In that study, 8.7% of children and adolescents were classified as overweight and 3.4% were categorized as obese. In addition, it was reported that female and urban pediatric populations generally displayed greater weight distributions compared to male and rural populations. According to a more recent population-based multicenter study in which Polish children between the ages of 7 and 9 years were enrolled, ~15% of children were categorized as overweight and ~4% were classified as obese [6].
In 1996, the European Childhood Obesity Group published a proposal advocating that a relative (age-adjusted) BMI for children be used [7]. Because BMI has been shown to increase from ~6 to ~17 years of age, it was deemed that for each country it was necessary to have age- and sex- specific BMI percentiles. Even when these criteria are taken into account, the prevalence of obesity amongst the pediatric population has often been underestimated [8,9].
It is generally accepted that greater levels of physical activity are associated with an enhanced health status. It has been reported that physical activity is inversely related to overweight and obesity in youth [10]. Physical fitness is generally considered to be the ability to perform daily tasks without fatigue and is commonly assessed with the use of the Eurofit test battery. Since its inception, the Eurofit test battery has been widely used throughout Europe to assess the effectiveness of physical education on child health and to measure the physical fitness of schoolchildren. The Eurofit test battery comprises numerous health- and performance-related fitness tests, including field tests to measure balance, cardio-respiratory (aerobic) endurance, muscular endurance (abdominal and upper body), flexibility, power, speed, agility, and strength [11,12].
There are several studies that show that obesity is associated with an inflammatory-like status. Furthermore, such a status is generally reported to be associated with early, long-lasting, and escalated obesity. Such a heightened inflammatory condition has been confirmed by the measurement of several biochemical markers [13]. Obesity should also be regarded as a direct risk of metabolic syndrome in children, because an increasing number of reports show biomarkers of cardiovascular disease already present even in young persons with highly increased body mass and body fat [14]. In addition, obesity-derived inflammation may lead to severe metabolic problems, including renal insufficiency [15], whereas reduction in body mass may lead to improvement of vital parameters [16]. It is already quite clear that physical exercise and fitness are important factors reducing risk of metabolic deterioration due to obesity.
Although a number of different types of inflammatory markers have been studied in obese subjects [17], IL6 and acute phase proteins such as alpha1-acid glycoprotein (AGP) and alpha1-antichymotrypsin (ACT) are relatively well studied and are implicated in various inflammatory pathologies. IL6 appears to play a pivotal role in the inflammatory cascade [13] and serum concentrations of AGP increase during infectious disorders, remodeling, and repair. In addition, the concentration of ACT has been shown to increase during tissue damage and necrosis [18]. Furthermore, the glycosylation profiles of AGP and ACT have been shown to be indicative of the physiological inflammatory status and in vivo cytokine balance [18]. Few studies have investigated the relationship between physical fitness, obesity, and inflammation in children and no such study had been conducted in Poland. The current study aimed at delineating this relationship by collating the measurements relating to the aforementioned inflammatory markers as well as the physical fitness of obese Polish adolescents.
Material and Methods
Study subjects
The cohort study group included 124 children and adolescents (63 females and 61 males). According to age, the investigated subjects were divided into 2 groups: aged 12–14 years old (23 females, 28 males) or 15–18 years old (40 females, 33 males). All subjects were described as post-pubertal.
Body mass index
A certified electronic scale model was used to record the mass of each subject to the nearest 0.1 kg. For each of these measurements, each subject wore light clothing and no shoes. The height of each child was measured to the nearest 0.01 m by use of a stadiometer. The BMI for each participant was calculated by using data obtained for the height and mass of each respective subject. Previously published age-specific BMI curves were used to determine whether a participating subject was overweight or obese. More specifically, subjects were divided according to the recorded BMI as compared to the results obtained for the Polish population: 90–97 percentiles or above 97 percentile [19,20].
Physical fitness
The physical fitness of each individual subject was measured using the Eurofit test battery. The following physical fitness tests were undertaken: flamingo balance (FLB); plate tapping (PLT); sit-and-reach (SAR); standing broad jump (SBJ); handgrip (HGR); sit-ups (SUP); bent-arm hang (BAH); shuttle run 10×5 m (SHR); and 20-m endurance shuttle run (ESR) [11,12,18]. The results obtained with the Eurofit test battery were compared with the previously published and readily available fitness percentiles for Polish adolescents [21]. All data are given in Table 1.
Table 1.
Inflammatory parameters for groups divided according to age and sex.
| Group: age | 12–14 | 15–18 | ||
|---|---|---|---|---|
| Sex | Female | Male | Female | Male |
| IL-6 (pg/L) | 1.21±0.3 | 1.7±0.3 | 2.3±0.7 | 1.5±04 |
| AGP (mg/L) | 968±279# | 1001±312# | 1252±354# | 888±212#,* |
| ACT (mg/L) | 376±90 | 344±97 | 486±101# | 474±210 |
Statistically significant difference (p<0.05) against healthy, non-obese peers;
statistically significant difference (p<0.05) between F/M.
Inflammatory markers
Blood samples were collected from each of the participating subjects. Following appropriate coagulation and centrifugation, the serum from each individual blood sample was isolated and used for further analysis. More specifically, the concentration of interleukin 6 (IL-6) was determined by ELISA (R&D, USA). In addition, the concentration of the following acute-phase proteins was determined: C-reactive protein (CRP); alpha-acid glycoprotein (AGP); and alpha1-antichymotrypsin (ACT). These latter concentrations were measured by the established methodology of immunoelectrophoresis according to Laurell. Antibodies and standard solutions were readily sourced from DakoCytomation® (Denmark). Additionally, the glycosylation profiles of AGP and ACT were analyzed by crossed-affinity immunoelectrophoresis with the ligand concanavalin A.
Reference values for acute-phase proteins in children were established earlier in the same laboratory, using the same methods. Sera from healthy children, undergoing routine laboratory testing, were used. No extra blood sampling took place.
Statistical analysis
For all variables, Gaussian normality was initially determined with the use of readily available statistical tests such as the Kolmogorov-Smirnov test for normality. For all generated datasets, average and standard deviation (SD) values were calculated. Data groups were compared using the t-test. The Pearson’s test was used to assess any correlation between measured variables. In all cases, results were considered statistically significant with a p value <0.05.
Results
Body mass index
Body mass index (BMI) calculated for the age groups was 29.1±3.8 for 12–14 years old females and 31.7±3.6 for 12–14 years old males. For the 15–18age group, it was 31.4±4.2 for females and 32.7±4.7 for males. When compared to percentile distribution for the Polish population, for females aged 12–14, eight were in the 90th–97th percentile and 15 were above the 97th percentile. For males, the numbers were 8 and 20, respectively. For the group aged 15–18, nine females were in the 90th–97th percentile and 31 were above the 97th percentile. For males, the numbers were 7 and 26, respectively. The vast majority of the studied population was found to have very high BMI, not necessarily higher with age.
Physical fitness tests
The physical fitness of all the enrolled subjects was assessed with the Eurofit test battery. The results, divided according to age, sex, and BMI percentiles are shown in Table 2, along with comparison with the previously published fitness percentile table for Polish adolescents [22].
Table 2.
Age, sex, and physical fitness results for the study population group, for subjects with BMI 90–97 percentile and below – for BMI >97 percentile.
| Age group | 12–14 | 15–18 | ||
|---|---|---|---|---|
|
| ||||
| Sex | Female | Male | Female | Male |
| Flamingo balance test FLB (n=) | 0 (↓) | 1 (↓) | 0 (↓) | 0 (↓) |
| 0 (↓) | 0 (↓) | 0 (↓) | 0 (↓) | |
|
| ||||
| Plate tapping test PLT (sec) | 17.7 (↑) | 18.5 (↑) | 12.0 (↑) | 14.2 (↑) |
| 17.6 (↑) | 17.2 (↑) | 13.2 (↑) | 11.6 (↑) | |
|
| ||||
| Sit-ups test SUP (n=) | 10 (↓) | 31 (↓) | 28 (↓) | 38 (↓) |
| 26 (↓) | 28 (↓) | 22 (↓) | 27 (↓) | |
|
| ||||
| Sit and reach test SAR (cm) | 19 (↑) | 9 (N) | 18 (↑) | 11 (N) |
| 15 (↑) | 21 (↑) | 19 (↑) | 19 (↑) | |
|
| ||||
| Standing broad jump test SBJ (cm) | 123 (↓) | 146 (↓) | 140 (↓) | 155 (↓) |
| 119 (↓) | 130 (↓) | 117 (↓) | 142 (↓) | |
|
| ||||
| Handgrip test HGR (kg) | 28.5 (↑) | 29.0 (N) | 31.8 (N) | 29.2 (N) |
| 26.0 (↑) | 31.0 (N) | 32.0 (N) | 45.8 (N) | |
|
| ||||
| Bent-arm hang test BAH (sec) | 0 (↓) | 3 (↓) | 0 (↓) | 0 (↓) |
| 0 (↓) | 0 (↓) | 0 (↓) | 0 (↓) | |
|
| ||||
| 10×5 m shuttle run SHR (sec) | 24.9 (↑) | 23.1 (↑) | 25.0 (↑) | 24.0 (↑) |
| 26.4 (↑) | 25.5 (↑) | 27.0 (↑) | 24.0 (↑) | |
|
| ||||
| 20 m endurance shuttle run ESR (min) | 18.5 (↓) | 37.5 (↓) | 34.0 (N) | 41.0 (↓) |
| 13.0 (↓) | 17.0 (↓) | 23.0 (↓) | 33.0 (↓) | |
Results were compared to previously published fitness percentile tables. ↑ – denotes greater than normal; ↓ – denotes less than normal; N – designates a normal value. Tests most affected by obesity are given in bold.
Females ages 12–14 had lower than normal scores in the SUP, SBJ, BAH, and ESR tests. In contrast, greater than normal performance scores were obtained in the FLB, PLT, SAR, HGR, and SHR tests. For boys aged 12–14 years, comparatively lower than normal performance scores were obtained in the SUP, SBJ, BAH, and ESR tests, and a greater than normal performance score was obtained in the FLB, PLT, SAR, and SHR tests.
For females ages 15 to 18 years, comparatively greater than normal scores were recorded in the FLB, PLT, SAR, HGR, and SHR tests, and boys in the same age group had greater than normal test scores in the PLT and SHR assessments. Lower than normal test scores were recorded for females ages15–18 in the SUP, SBJ, and BAH fitness tests. Male subjects in the same age range had lower than normal performance scores in the SUP, SBJ, BAH, and ESR tests.
An overview of the results for the Eurofit test battery showed that all of the test subjects experienced only minor difficulty in performing the flexibility assessment (SAR) test. In contrast, both boys and girls found the tests measuring explosive leg power (SBJ), functional strength (BAH), and the ability to accelerate and change direction (SHR) very difficult. Male subjects in all age groups generally had performance scores for the cardiorespiratory endurance (ESR) test that were well below the normal values previously published for Polish boys. With regards to the static arm strength (HGR) test, girls from both age groups had superior performance scores compared to the fitness percentile tables for Polish adolescents and scores for all male subjects were within normal range.
The table also shows Eurofit test elements most affected by obesity (ie, those in which the results for subjects in the 90th–97th percentile were better than for those above the 97th percentile. However, the differences were minor and the fitness of all investigated subjects was impaired by obesity.
Inflammatory markers
For all studied age groups and sexes, the average IL-6 concentration was only slightly greater than the reported normal average value of 1.77 pg/mL (Tables 1 and 3). Due to the relatively large standard deviation of all studied subgroups, no statistically significant differences could be detected.
Table 3.
Sex, age, concentration of selected inflammatory markers as well as the distribution profile of glycosylated acute phase proteins for study population subgroups.
| Group: age | 12–14 | 15–18 | ||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Female | Male | Female | Male | ||||
| Percentile of BMI | 90–97% | >97% | 90–97% | >97% | 90–97% | >97% | 90–97% | >97% |
| IL-6 (pg/L) | 1.2 | 1.1 | 1.8 | 1.7 | 2.2 | 2.3 | 1.7 | 1.3 |
| AGP (mg/L) | 1108±321 | 908±250 | 1578±327 | 940±250 | 930±223 | 1333±281 | 904±238 | 896±260 |
| W0 (%) | 44 | 44 | 46 | 41 | 43 | 39 | 41 | 38 |
| W1 (%) | 43 | 44 | 40 | 43 | 39 | 43 | 44 | 43 |
| W2 (%) | 12 | 10 | 13 | 13 | 15 | 15 | 12 | 16 |
| W3 (%) | 1 | 1 | 1 | 3 | 2 | 2 | 2 | 3 |
| ACT (mg/L) | 382±174 | 373±97 | 504±139 | 328±100 | 371±136 | 515±187 | 494±130 | 468±118 |
| A1 (%) | 26 | 28 | 18 | 30 | 24 | 26 | 24 | 23 |
| A2 (%) | 31 | 33 | 26 | 32 | 40 | 30 | 31 | 30 |
| A3 (%) | 22 | 21 | 26 | 23 | 20 | 23 | 26 | 23 |
| A4 (%) | 13 | 10 | 23 | 10 | 13 | 16* | 13 | 18 |
| A5 (%) | 8 | 7 | 7 | 4 | 2 | 5 | 5 | 5 |
Normal serum values for AGP and ACT have been reported to be 800±100 mg/L and 400±50 mg/L, respectively [23]. With regards to AGP, the concentration of the investigated acute-phase protein was higher than normal in all studied subgroups. A number of AGP variants are known to exist and their distribution in percentage is as follows: W0 (43%), W1 (45%), W2 (12%), and W3 (2%) [23,24].
With regards to ACT, the serum concentration of this protein was within the normal range. For ACT, the following variants and respective distributions have been reported: A1 (25%), A2 (24%), A3 (26%), and A4 (25%) [23,24]. The glycosylation profile of ACT was altered in almost all children.
The distribution of distinctly glycosylated variants of AGP and ACT is shown in Figure 1. The increase of percentages of variants strongly reactive with concanavalin A is noticed mainly for males.
Figure 1.
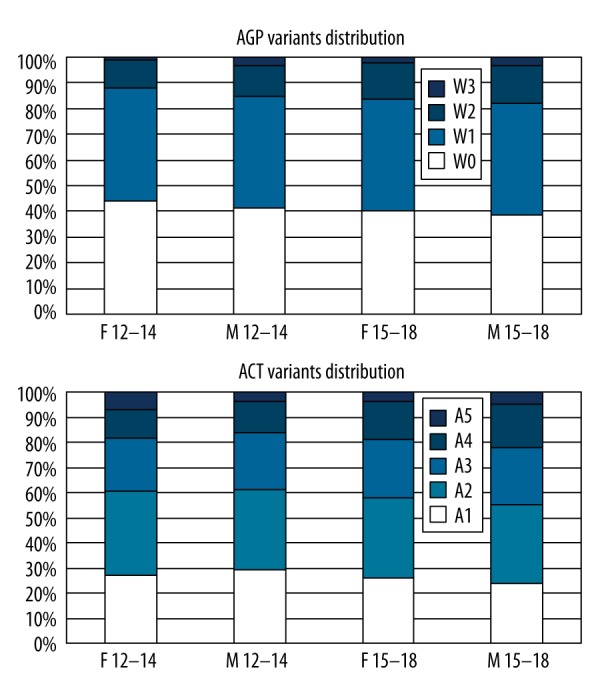
Dstribution of distinctly glycosylated variants of acid alpha1-glycoprotein (AGP) and antichymotrypsin (ACT) in children divided according to age and sex. Variants of lower numbers are less Concanavalin A reactive, variants with higher numbers are more reactive.
Twelve to 14 years age group
When the concentration values of the examined inflammatory markers in the 12–14 years age group were compared, no statistically significant differences could be discerned between the two sexes. When children within this age range were subdivided according to BMI percentages, no statistically significant differences between the concentration values of inflammatory markers could be observed, but a tendency to lower acute phase proteins concentrations could be seen in males. All correlations observed between inflammatory markers and physical fitness measures in this group are shown in Table 4.
Table 4.
Numerical correlation values of inflammatory and physical fitness measures. Only statistically significant (p<0.05) correlations are given.
| FLB | PLT | SUP | SAR | SBJ | HGR | BAH | SHR | ESR | |
|---|---|---|---|---|---|---|---|---|---|
| IL-6 | −0.62## | −0.76** | 0.73** | ||||||
| AGP | 0.46* | 0.53## | −0.48# | ||||||
| W0% | 0.61** | −0.49## | |||||||
| W1% | 0.44# | ||||||||
| W2% | −0.67** | −0.53## | |||||||
| W3% | −0.43** | 0.56* 0.46## |
|||||||
| ACT | 0.50## | 0.63# | |||||||
| A1% | |||||||||
| A2% | |||||||||
| A3% | −0.48** | ||||||||
| A4% | −0.46## | ||||||||
| A5% | −0.47** |
Female 12–14 years;
female 15–18 years;
male 12–14 years;
male 15–18 years.
Fifteen to 18 years age group
In the 15–18 years age group, the IL-6 concentration and AGP serum concentration value were significantly greater in females compared to males (p=0.02). Furthermore, the value was significantly greater in the 15–18 years age group compared to younger subjects. Statistically significant increases in ACT concentration occurred across both sexes when the values in the 15–18 years age group were compared to those in the younger groups. The concentration of both investigated acute-phase proteins did not differ in healthy individuals from values observed previously [25], but were increased in obese children, particularly in girls.
The glycosylation profile of AGP in healthy children was not equal to previously reported images observed in adults: the percentage of the W0 variant, non-reactive to ConA, was higher, and the W3 variant, non-present in healthy adults, was observed. Obesity was accompanied with a profile typical for acute inflammatory conditions in adults, and the intensity of this feature increased with BMI.
In healthy children aged 12–14 years, ACT concentration was lower than previously reported in adults; this feature was also reported earlier by us [25]. An increase in ACT concentration takes place first in the post-pubertal period, thus concentrations observed in healthy teenagers ages 15–18 did not differ from those observed in adults. Obesity in general increased ACT concentration, but higher BMI percentile was not associated with higher values.
The glycosylation profile of ACT showed in healthy children an extra A5 variant, observed in adults only during particularly acute inflammatory conditions (e.g., trauma, burn, severe bacterial infection). Its percentage increased in obese children. The most expressed shift towards acute inflammatory image (increase of ConA reactivity) was noticed for girls and boys ages 12–14 years with BMI in the 90–97 percentiles.
Fitness and inflammation
When physical fitness tests results were compared to the presence of inflammatory markers, a number of significant correlations were found (Table 4).
Significant (p<0.05) correlations were observed between low physical fitness scores and increased inflammatory parameters. Negative correlations were observed for FLB, BAH, and SBJ tests, whereas more positive correlations were found for SAR and SUP tests. There were no correlations at all for SHR, HGR, and ESR tests. Static strength and endurance were not affected by obesity.
Discussion
The Eurofit fitness test battery was developed as a standardized test for measuring the physical fitness of schoolchildren [21]. Deforche et al reported that the physical fitness scores of non-obese subjects were generally superior to those of obese participants [26]. This is concordant with the results of the current study, in which lower than normal fitness scores were generally recorded for overweight and obese Polish children compared to those normally achieved. This finding was generally consistent across all studied age groups. Ara et al. (2010) reported that the level of physical fitness was inversely related to adiposity [27] and that adipose tissue deposition is reduced when health is characterized by average or above average physical fitness measures.
The results of the current study indicate that children with increased adiposity are characterized by a somewhat abnormal immune status. The IL-6 concentration was only slightly greater than normal (Tables 1 and 3) in most studied individuals, but the AGP concentration was significantly higher than normal in all of the population subgroups. Furthermore, the glycosylation profiles of AGP and ACT were characteristic of the presence of a systemic immune reaction. Although a number of reports have been published describing the presence of various acute-phase proteins in the serum of obese adults [28], such data is scarcer for children or adolescents. The collated results indicate that an increased level of fatness may result in a general and systemic inflammatory-like status and this is strengthened by correlations between the various inflammatory markers, physical fitness measures, and adiposity assessments. It has been reported that various inflammatory markers are increased in overweight or obese patients with metabolic syndrome [17,29]. In the current study, the degree of adiposity appeared to have a negative impact on physical fitness scores; these scores, in turn, appeared to be correlated in some instances with the investigated acute-phase proteins.
The concentration of AGP correlated positively with SUP (showing strength of the trunk or muscle endurance), but negatively with FLB or PLT (showing balance and speed). It could be concluded that obesity-driven inflammatory status had greater effect on the neural/psychic elements of fitness, and less effect on the pure muscle component. Handgrip was stronger in most investigated subjects, showing the known positive impact of larger body mass on strength. Higher muscle strength was previously reported in overweight individuals [30].
In general, explosive strength was negatively correlated with inflammatory parameters, whereas static strength was positively correlated. No influence on endurance could be shown; the results obtained were very low in comparison to the Polish population with normal body mass.
No differences due to BMI were observed, but all investigated subjects had high BMI and showed equal alterations in inflammatory markers, as compared to healthy non-overweight or obese subjects.
No differences according to sex were observed in the 12–14 age group, whereas for the 15–18 age group AGP concentration differed significantly (p=0.01). Lower value does not necessarily mean better status, as a more acute inflammatory pattern of both AGP and ACT variants was observed in the older male group. Acute-phase proteins may play an anti-inflammatory role and non-increased concentration may mean no protection. Worse results of fitness tests in these individuals may also indicate that compensation of excessive fat tissue is inadequate in older males.
Results of some Eurofit test elements (ESR and SHR) may to some extent be compared to cardiovascular fitness (CVF), reported by several authors. In overweight or obese children, CVF correlated negatively with C-reactive protein (CRP) concentration, C3, and C4 [31,32].
CRP was also reported to correlate negatively with adiposity (BMI or body fat) [33]. Other studies showed reduction of CRP concentration upon regular physical exercise leading to body mass reduction [34]. In our studies CRP was not investigated, instead we measured 2 other acute-phase proteins concentrations, which were also reported to be directly IL-6 dependent. Both showed increased concentrations, dependent on overweight degree and their concentrations correlated positively with increasing muscle strength. It has been postulated that IL-6 may not only be regarded as a pro-inflammatory agent, but that its biological effect may in fact be beneficial, causing increased insulin sensitivity of the muscles and leading to production of anti-inflammatory substances such as acute-phase proteins [35]. IL-6 may be produced in the muscles themselves, increasing glucose availability via stimulation of insulin production [34]. Thus, the inflammatory markers are probably to some extent combined with increased work load needed to move increased body mass in overweight children. It seems important, however, that after a given level of disturbances is noticed, no compensation is possible and inflammatory reaction starts to be potentially dangerous.
Fit though obese children showed normal inflammatory values, whereas obese and non-fit children had increased levels of inflammatory parameters [33,36]. In our studies overweight girls aged 12–14 years showed no deterioration of physical fitness, whereas obese boys aged 15–18 were no more fit and showed high inflammatory values. Thus, both these parameters should always be taken into consideration when describing inflammatory markers in overweight children. As long as physical fitness is not affected, increased concentrations of IL-6, CRP, or other acute-phase proteins may have homeostatic significance as the sign of an anti-inflammatory effect of IL-6. If fitness deteriorates, body mass probably increased to the level of decompensation, and then alterations in the glycosylation profiles of acute phase proteins may be observed. This feature should be regarded as potentially harmful.
Conclusions
In conclusion, we found that the degree of obesity (BMI) and the age influence the physical fitness scores of children. More specifically, an older child characterized by a large BMI value will have suboptimal physical fitness test scores. In general, such a child is differentiated by acute-phase proteins that are glycosylated to a pattern indicative of an inflammatory status. The Eurofit and protein analysis results of female subjects appeared to be less influenced by the degree of fatness. This may indicate that girls are more resistant to the negative effect of an increased adiposity status. Because the adiposity of children appears to be related to their inflammatory status, this finding may explain the future emergence of various immune-type pathologies and diseases in such individuals.
Ethics statement
The performed study involved human subjects for measurements and observations. The research was performed after obtaining approval from the Bioethics Committee, Institutional Review Board at Poznan University of Medical Sciences issued on 3 April 2008.
The authors declare that all identifiable human data used for tests and examinations in the study underwent research procedures in accordance with the ethics standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983.
Acknowledgements
The authors thank the Proper Medical Writing (infrared group s.c.) for the technical and language assistance in the preparation of this paper.
Footnotes
Source of support: Departmental sources
References
- 1.Ogden CL, Carroll MD, Curin LR, et al. Prevalence of overweight and obesity in the United States 1999–2004. JAMA. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 2.WHO Technical Report Series No. 894. Geneva: 2000. Obesity: Preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 3.Ogden CL, Flegan KM, Carroll MD, et al. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–32. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 5.Oblacińska A, Wrocławska M, Wojnarowska B. The prevalence of overweight and obesity amongst Polish school children. Medical care of children suffering from those conditions. Ped Pol. 1997;72:241–45. [Google Scholar]
- 6.Małecka-Tendera E, Klimek K, Matusik P, et al. Obesity and overweight prevalence in Polish 7-to 9-year-old children. Obes Res. 2005;13:964–68. doi: 10.1038/oby.2005.112. [DOI] [PubMed] [Google Scholar]
- 7.Poskitt EM. Defining childhood obesity: the relative body mass index (BMI) Acta Paediatr. 1995;84:961–63. doi: 10.1111/j.1651-2227.1995.tb13806.x. [DOI] [PubMed] [Google Scholar]
- 8.Reilly JJ. Descriptive epidemiology and health consequences of childhood obesity. Best Prac Res Clin Endocrinol Metab. 2005;19:327–41. doi: 10.1016/j.beem.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Bundak R, Furman A, Gunoz H, et al. Body mass index references for Turkish children. Acta Paediatr. 2006;95:194–98. doi: 10.1080/08035250500334738. [DOI] [PubMed] [Google Scholar]
- 10.Lubans RD, Boreham CA, Kelly P, et al. The relationship between active travel to school and health-related fitness in children and adolescents: a systematic review. Int J Behav Nutr Phys Act. 2011;8:5. doi: 10.1186/1479-5868-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomkinson GR, Olds TS, Borms J. Who Are the Eurofittest? Med Sport Sci. 2007;50:104–28. doi: 10.1159/000101355. [DOI] [PubMed] [Google Scholar]
- 12.Adam C, Klissouras V, Ravazzolo M, et al. Eurofit: European Test of Physical Fitness. Council of Europe, Committee for the Development of Sport; Rome: 1988. [Google Scholar]
- 13.Popko K, Gorska E, Stelmaszczyk-Emmel A, et al. Proinflammatory cytokines Il-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res. 2010;2:120–22. doi: 10.1186/2047-783X-15-S2-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss R, Dziura J, Burgert TS. Obesity and the Metabolic Syndrome in Children and Adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 15.Naumnik B, Mysliwiec M. Renal consequences of obesity. Med Sci Monit. 2010;16(8):RA163–70. [PubMed] [Google Scholar]
- 16.Haspicova M, Milek D, Siklova-Vitkova M, et al. Post-prandial endothelial dysfunction is ameliorated following weight loss in obese premenopausal women. Med Sci Monit. 2011;17(11):CR634–39. doi: 10.12659/MSM.882048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallianou NG, Evangelopoulos AA, Panagiotakos DB, et al. Associations of acute-phase reactants with metabolic syndrome in middle-aged overweight or obese people. Med Sci Monit. 2010;16:CR56–60. [PubMed] [Google Scholar]
- 18.Markuszewski J, Wierusz-Kozłowska M, WoŸniak W, et al. Early acute phase response following total hip replacement. Ortop Traumatol Rehabil. 2009;4:324–32. [PubMed] [Google Scholar]
- 19.Kułaga Z, Litwin M, Tkaczyk M, et al. Polish 2010 growth references for school-aged children and adolescents. Eur J Pediatr. 2011;170(5):599–609. doi: 10.1007/s00431-010-1329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–43. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Council of Europe. EUROFIT – European Test of Physical Fitness. 2nd ed. Strassbourg: 1993. [Google Scholar]
- 22.Stupnicki R, Przewęda R, Milde K. Percentile references curves for physical fitness measured by Eurofit tests in Polish youths. AWF Warszawa. 2003 [Google Scholar]
- 23.Pawlaczyk M, Sobieska M, Wiktorowicz K. Generalised inflammatory reaction present in plaque stage of mycosis fungoides. Skin Cancer. 2001;16:115–22. [Google Scholar]
- 24.Lialikau SA, Haurylik LL, Sobieska M, et al. Acute phase proteins serum concentrations in children are related to urinary iodine excretion. Rocz AM Białyst. 2005;50:279–83. [PubMed] [Google Scholar]
- 25.Pucher B, Sobieska M, Steiner I, et al. Evaluation of chosen parameters of acute-phase reaction in children during pseudocroup according to their age. Otolar Pol. 2006;60:743–46. [PubMed] [Google Scholar]
- 26.Deforche B, Lefevre J, De Bourdeaudhuij I, et al. Physical fitness and physical activity in obese and nonobese Flemish youth. Obes Res. 2003;11:434–41. doi: 10.1038/oby.2003.59. [DOI] [PubMed] [Google Scholar]
- 27.Ara I, Sanchez-Villegas A, Vicente-Rodriguez G, et al. Physical fitness and obesity are associated in a dose-dependent manner in children. Ann Nutr Metab. 2010;57:251–59. doi: 10.1159/000322577. [DOI] [PubMed] [Google Scholar]
- 28.Heliövaara MK, Teppo AM, Karonen SL, et al. Plasma IL-6 concentration is inversely related to insulin sensitivity, and acute-phase proteins associate with glucose and lipid metabolism in healthy subjects. Diabetes Obes Metab. 2005;7:729–36. doi: 10.1111/j.1463-1326.2004.00463.x. [DOI] [PubMed] [Google Scholar]
- 29.Chyrek R, Bogdanki P, Pupek-Musialik D, et al. Evaluation of selected acute phase proteins in patients type 2 diabetes. Farm Wspł. 2008;1:5–10. [Google Scholar]
- 30.Adamo KB, Sheel AW, Onywera V, et al. Child obesity and fitness levels among Kenyan and Canadian children from urban and rural environments: A KIDS-CAN Research Alliance Study. Int J Pediatr Obes. 2010;6(2–2):e225–32. doi: 10.3109/17477166.2010.543683. [DOI] [PubMed] [Google Scholar]
- 31.Ortega FB, Ruiz JR, Castillo MJ. Physical fitness in childhood and adolescence: a powerful marker of health. Fitness as a health marker in young people? Int J Obes. 2008;32(1):1–11. doi: 10.1038/sj.ijo.0803774. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz JR, Ortega FB, Warnberg J, et al. Associations of low-grade inflammation with physical activity, fitness and fatness in prepubertal children; the European Youth Heart Study. Int J Obes. 2007;31:1545–51. doi: 10.1038/sj.ijo.0803693. [DOI] [PubMed] [Google Scholar]
- 33.Parrett AL, Valentine RJ, Arngrimsson SA, et al. Adiposity, Activity, Fitness, and C-Reactive Protein in Children. Med Sci Sports Exerc. 2010;42:1981–86. doi: 10.1249/MSS.0b013e3181e0355e. [DOI] [PubMed] [Google Scholar]
- 34.Kasapis Ch, Thompson PD. The Effects of Physical Activity on Serum C-Reactive Protein and Inflammatory Markers A Systematic Review. J Am Coll Cardiol. 2005;10:1563–69. doi: 10.1016/j.jacc.2004.12.077. [DOI] [PubMed] [Google Scholar]
- 35.Mathur N, Pedersen BK. Exercise as a Mean to Control Low-Grade Systemic Inflammation. Mediators Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin H, Korsten-Reck U, Wolfarth B, et al. Low-grade systemic inflammation in overweight children: impact of physical fitness. Exerc Immunol Rev. 2004;10:66–74. [PubMed] [Google Scholar]


