Abstract
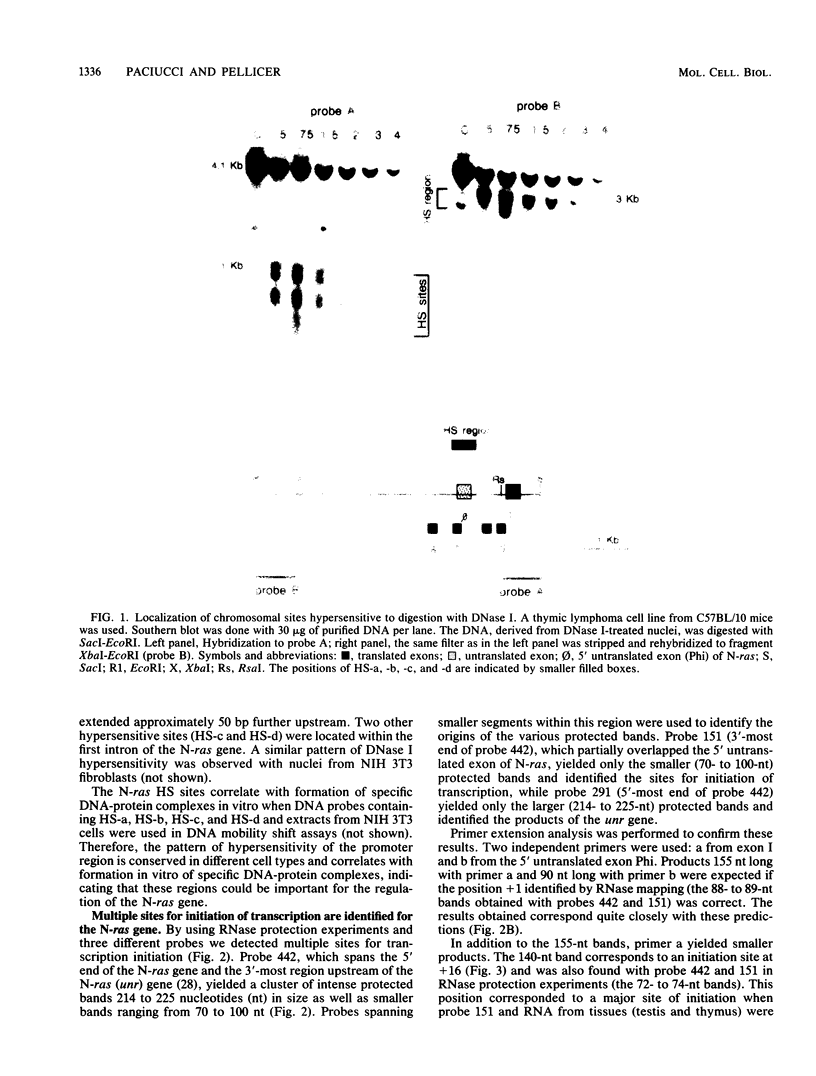
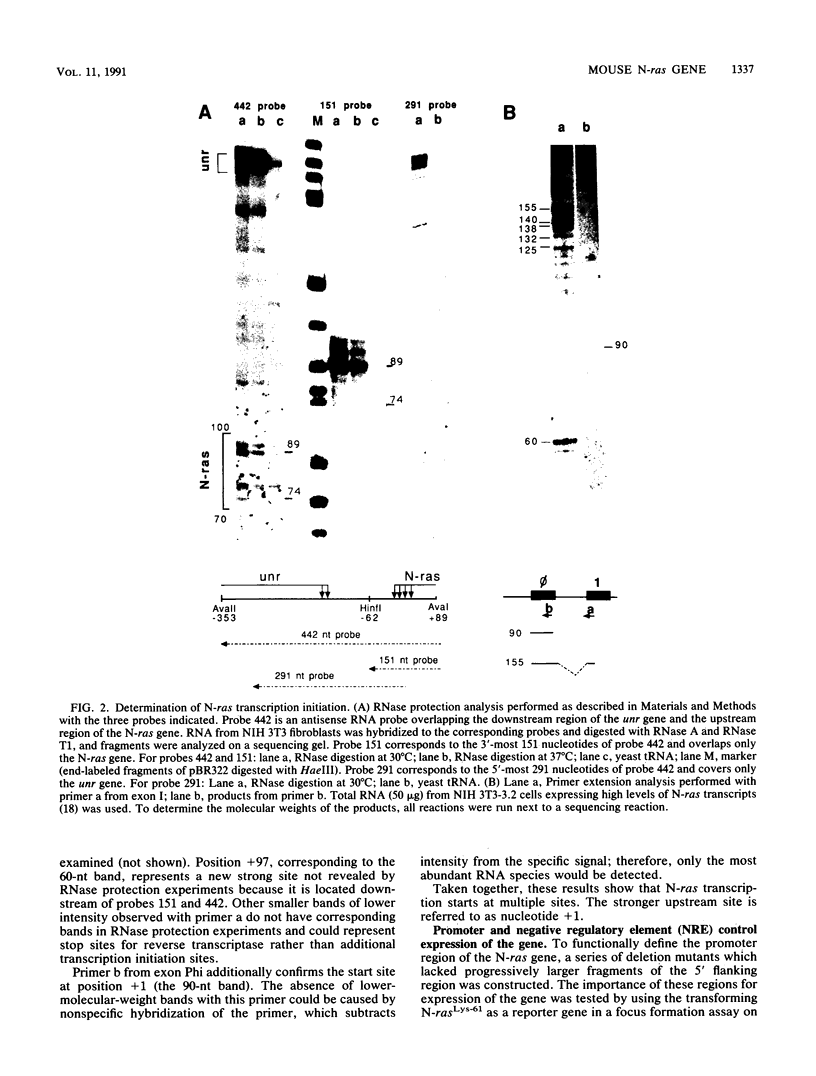
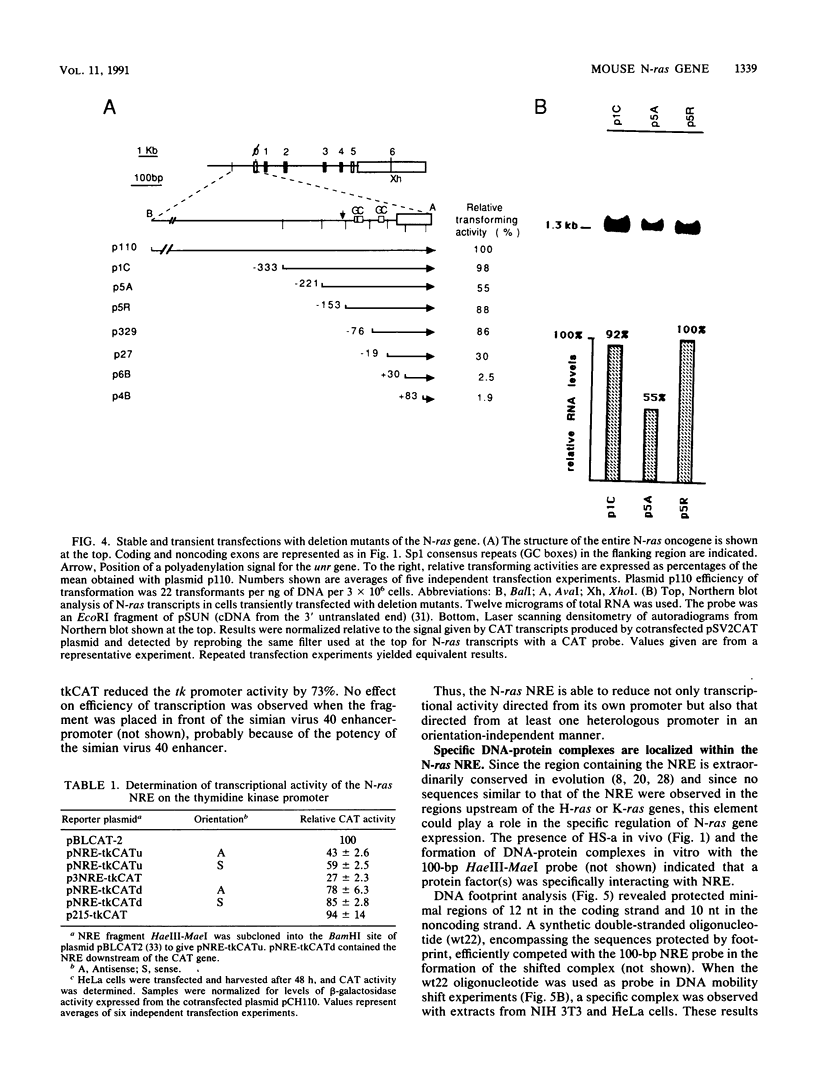
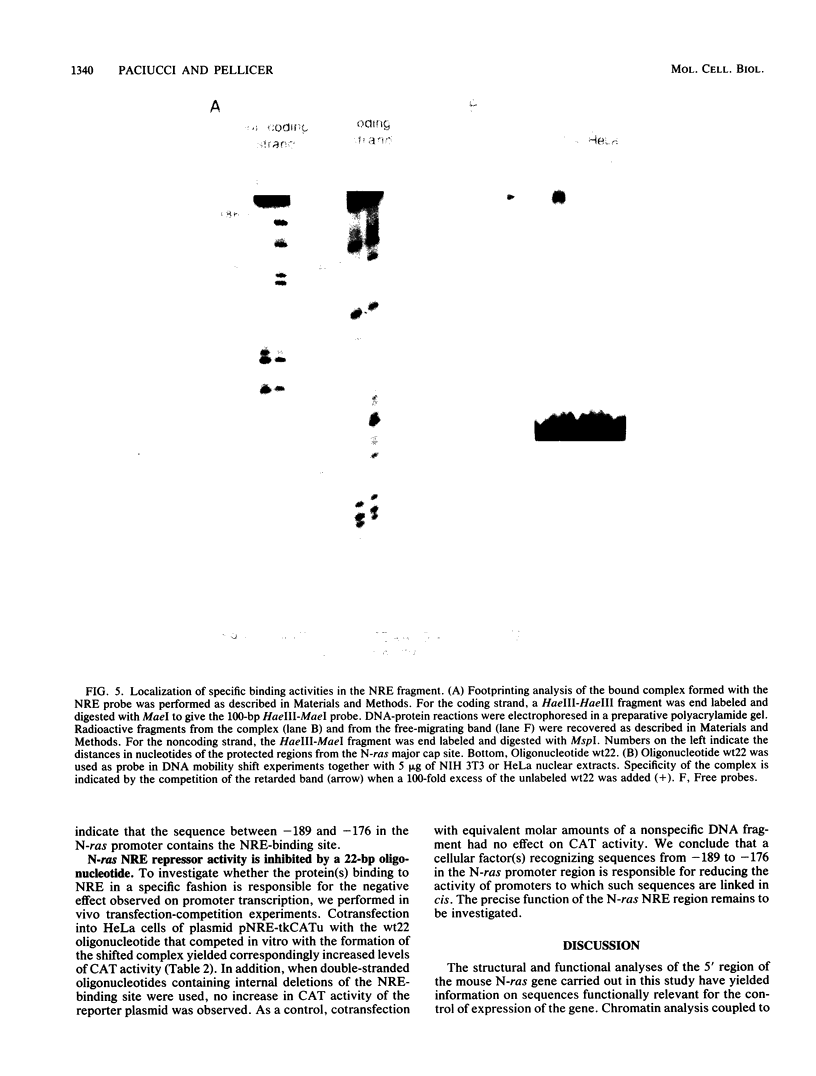
The 5' flanking region of the mouse N-ras gene was investigated to determine the elements governing transcriptional activity of the gene. The promoter did not contain typical TATA or CCAAT boxes, and according to primer extension and RNase protection analyses, transcription started at several sites. These assays also confirmed the short nucleotide distance interposed between the N-ras transcription unit and the previously described upstream unr gene. Chromatin studies performed by digestion of nuclei with DNase I revealed the presence of four hypersensitive sites: a, b, c, and d. Deletion mutagenesis of the 5' flanking region revealed sequences responsible for both promotion and inhibition of transcription. These sequences resided within 230 bp upstream of the transcription initiation site. Hypersensitive site b colocalized with the 76-bp segment with promoter activity. The negative regulatory element at position -180 colocalized with hypersensitive site a, was active on the N-ras promoter in stable as well as transient assays, and down-regulated the heterologous herpes simplex virus thymidine kinase promoter. Footprint analysis and in vivo transfection-competition experiments indicated that a trans-acting factor is responsible for the negative effect on transcription. The interaction between the cis-acting negative regulatory element and the promoter region may play a role in the tissue- and developmental-stage-specific patterns of expression of the N-ras gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baniahmad A., Muller M., Steiner C., Renkawitz R. Activity of two different silencer elements of the chicken lysozyme gene can be compensated by enhancer elements. EMBO J. 1987 Aug;6(8):2297–2303. doi: 10.1002/j.1460-2075.1987.tb02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bond V. C., Wold B. Poly-L-ornithine-mediated transformation of mammalian cells. Mol Cell Biol. 1987 Jun;7(6):2286–2293. doi: 10.1128/mcb.7.6.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Furth M. E., Scolnick E. M., Lowy D. R. Tumorigenic transformation of mammalian cells induced by a normal human gene homologous to the oncogene of Harvey murine sarcoma virus. Nature. 1982 Jun 10;297(5866):479–483. doi: 10.1038/297479a0. [DOI] [PubMed] [Google Scholar]
- Chang H. Y., Guerrero I., Lake R., Pellicer A., D'Eustachio P. Mouse N-ras genes: organization of the functional locus and of a truncated cDNA-like pseudogene. Oncogene Res. 1987 Jul;1(2):129–136. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiswell D. J., Gillespie D. A., Wyke J. A. The changes in proviral chromatin that accompany morphological variation in avian sarcoma virus-infected rat cells. Nucleic Acids Res. 1982 Jul 10;10(13):3967–3980. doi: 10.1093/nar/10.13.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doniger J., DiPaolo J. A. Coordinate N-ras mRNA up-regulation with mutational activation in tumorigenic guinea pig cells. Nucleic Acids Res. 1988 Feb 11;16(3):969–980. doi: 10.1093/nar/16.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Maniatis T. Overlapping positive and negative regulatory domains of the human beta-interferon gene. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1447–1451. doi: 10.1073/pnas.85.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross D. S., Garrard W. T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Loss of the normal N-ras allele in a mouse thymic lymphoma induced by a chemical carcinogen. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7810–7814. doi: 10.1073/pnas.82.23.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Wong H., Pellicer A., Burstein D. E. Activated N-ras gene induces neuronal differentiation of PC12 rat pheochromocytoma cells. J Cell Physiol. 1986 Oct;129(1):71–76. doi: 10.1002/jcp.1041290111. [DOI] [PubMed] [Google Scholar]
- Hall A., Brown R. Human N-ras: cDNA cloning and gene structure. Nucleic Acids Res. 1985 Jul 25;13(14):5255–5268. doi: 10.1093/nar/13.14.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Herbst R. S., Boczko E. M., Darnell J. E., Jr, Babiss L. E. The mouse albumin enhancer contains a negative regulatory element that interacts with a novel DNA-binding protein. Mol Cell Biol. 1990 Aug;10(8):3896–3905. doi: 10.1128/mcb.10.8.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman E. K., Trusko S. P., Freeman N., George D. L. Structural and functional characterization of the promoter region of the mouse c-Ki-ras gene. Mol Cell Biol. 1987 Jul;7(7):2592–2596. doi: 10.1128/mcb.7.7.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkawa H., Masahashi W., Hashimoto S., Hashimoto-Gotoh T. Identification of the principal promoter sequence of the c-H-ras transforming oncogene: deletion analysis of the 5'-flanking region by focus formation assay. Mol Cell Biol. 1987 Aug;7(8):2933–2940. doi: 10.1128/mcb.7.8.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Kadonaga J. T., Tjian R., Brady J. N., Merlino G. T., Pastan I. Binding of the Sp1 transcription factor by the human Harvey ras1 proto-oncogene promoter. Science. 1986 Jun 13;232(4756):1410–1413. doi: 10.1126/science.3012774. [DOI] [PubMed] [Google Scholar]
- Jeffers M., Paciucci R., Pellicer A. Characterization of unr; a gene closely linked to N-ras. Nucleic Acids Res. 1990 Aug 25;18(16):4891–4899. [PMC free article] [PubMed] [Google Scholar]
- Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987 Jun 19;49(6):741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Leon J., Guerrero I., Pellicer A. Differential expression of the ras gene family in mice. Mol Cell Biol. 1987 Apr;7(4):1535–1540. doi: 10.1128/mcb.7.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay I. A., Marshall C. J., Calés C., Hall A. Transformation and stimulation of DNA synthesis in NIH-3T3 cells are a titratable function of normal p21N-ras expression. EMBO J. 1986 Oct;5(10):2617–2621. doi: 10.1002/j.1460-2075.1986.tb04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Muglia L., Rothman-Denes L. B. Cell type-specific negative regulatory element in the control region of the rat alpha-fetoprotein gene. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7653–7657. doi: 10.1073/pnas.83.20.7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Ko M., Ogura A., Liu D. G., Amano T., Takano T., Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985 Nov 7;318(6041):73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Propst F., Rosenberg M. P., Vande Woude G. F. Proto-oncogene expression in germ cell development. Trends Genet. 1988 Jul;4(7):183–187. doi: 10.1016/0168-9525(88)90073-x. [DOI] [PubMed] [Google Scholar]
- Pulciani S., Santos E., Long L. K., Sorrentino V., Barbacid M. ras gene Amplification and malignant transformation. Mol Cell Biol. 1985 Oct;5(10):2836–2841. doi: 10.1128/mcb.5.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers E. F., Yang J. Q., Marcu K. B. A negative transcriptional control element located upstream of the murine c-myc gene. EMBO J. 1986 May;5(5):899–904. doi: 10.1002/j.1460-2075.1986.tb04301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts M. H., Levinson A. D. High-level expression of c-H-ras1 fails to fully transform rat-1 cells. Mol Cell Biol. 1988 Apr;8(4):1460–1468. doi: 10.1128/mcb.8.4.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer E., Boissier F., Py M. C., Cohen G. N., Zakin M. M. Cell type-specific expression of the human transferrin gene. Role of promoter, negative, and enhancer elements. J Biol Chem. 1989 May 5;264(13):7153–7160. [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Sorrentino V., McKinney M. D., Giorgi M., Geremia R., Fleissner E. Expression of cellular protooncogenes in the mouse male germ line: a distinctive 2.4-kilobase pim-1 transcript is expressed in haploid postmeiotic cells. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2191–2195. doi: 10.1073/pnas.85.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Subramaniam A., Gulick J., Robbins J. Analysis of the upstream regulatory region of a chicken skeletal myosin heavy chain gene. J Biol Chem. 1990 Aug 15;265(23):13986–13994. [PubMed] [Google Scholar]
- Winoto A., Baltimore D. Alpha beta lineage-specific expression of the alpha T cell receptor gene by nearby silencers. Cell. 1989 Nov 17;59(4):649–655. doi: 10.1016/0092-8674(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto F., Perucho M. Characterization of the human c-K-ras gene promoter. Oncogene Res. 1988 Sep;3(2):125–130. [PubMed] [Google Scholar]