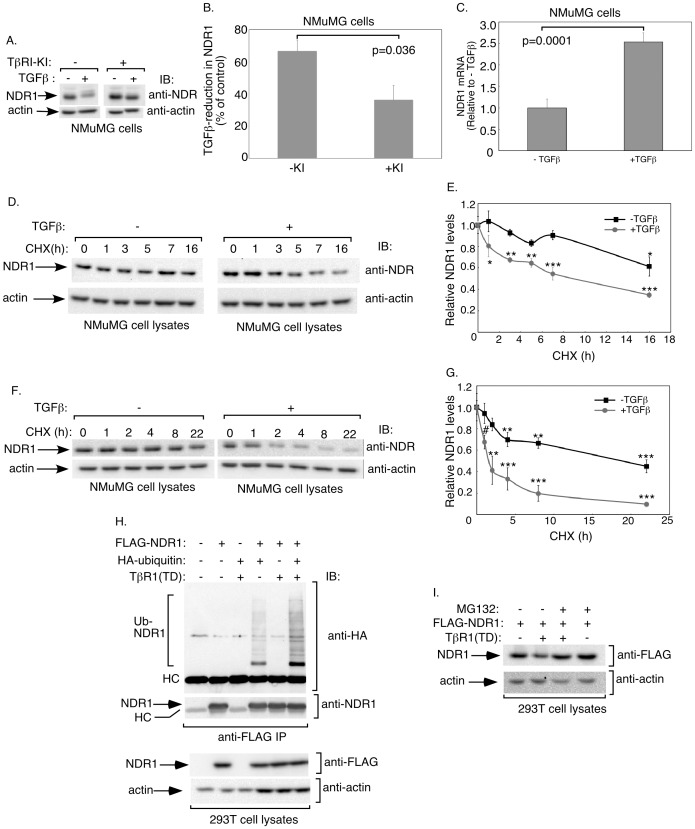
Figure 6. TGFβ signaling promotes NDR1 turnover.
A. Lysates of untreated or 48h-TGFβ-treated NMuMG cells in the absence or presence of the TGFβ type I receptor kinase inhibitor SB431542 (KI) were subjected to immunoblotting with the NDR1 or actin antibody. B. Protein abundance of NDR1 and actin in immunoblots, including those shown in A, were quantified and percent reduction of NDR1 (normalized to actin) in response to TGFβ was analyzed. Data are presented as the mean+SEM (n = 4) of percent decrease in protein abundance of NDR1 in NMuMG cells in response to TGFβ. TGFβ treatment decreased the protein abundance of NDR1 in NMuMG cells. C. TGFβ does not repress NDR1 mRNA expression. RNA extracts from untreated or 48h-TGFβ-treated NMuMG cells were analyzed by quantitative RT-PCR for NDR1 and GAPDH mRNA abundance. Data are presented as the mean+SEM (n = 3) of relative mRNA abundance of NDR1 in NMuMG cells. TGFβ did not reduce relative abundance of NDR1 mRNA. Significant differences are indicated in B and C as determined by unpaired, two-tailed t-test. D. Lysates of NMuMG cells left untreated or incubated with 10 µg/ml cycloheximide for different times, alone or together with 100 pM TGFβ, were subjected to immunoblotting using the NDR1 or actin antibody. E. Protein abundance of NDR1 in immunoblots, including the one shown in Figure 6D, were quantified and normalized to actin. Data are presented as the mean±SEM (n = 3) of normalized protein abundance of NDR1 expressed relative to that at time 0 for the respective minus or plus TGFβ group. Data interpolation indicated that NDR1's half-life was greater than 16 h. TGFβ reduced NDR1's half-life to approximately 9 h. F. Lysates of untreated or 24 h-TGFβ-preincubated NMuMG cells followed by exposure to cycloheximide for different time points, were subjected to immunoblotting using NDR1 or actin antibody. G. Protein abundance of NDR1 in immunoblots as described and including the one shown in Figure 6F was quantified as described in E. Data are presented as the mean±SEM (n = 4) of relative NDR1 levels. TGFβ reduced the half-life of NDR1 from greater than 16 h to approximately 2.5 h. H. TGFβ signaling enhances the ubiquitination of NDR1. Lysates of 293T cells expressing FLAG-NDR1, HA-ubiquitin, and constitutively active TGFβ type I receptor, were subjected to immunoprecipitation using the FLAG antibody, followed by immunoblotting with the HA or NDR1 antibody. Cell lysates were also immunoblotted with the FLAG or actin antibody. HC refers to the heavy chain of the FLAG antibody. I. Lysates of 293T cells transfected with FLAG-NDR1 alone or together with constitutively active TGFβ type I receptor and treated without or with 0.5 µM MG132 (Sigma) for 7 hours were subjected to immunoblotting with the FLAG or actin antibody. *, **, or *** in E and G indicates significant difference from respective control at P<0.05, p<0.01, or p<0.001, respectively (ANOVA). # indicates significant difference from control (p<0.05, unpaired, one tailed t-test).